All News
APPs in the News (12.5.2025)
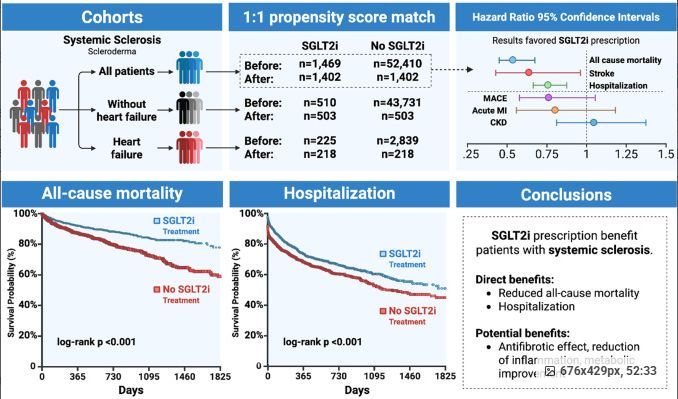
Dr. Jack Cush reviews the news and journal reports from RheumNow.com, including info on scleroderma, dermatomyositis and malignancy, rheumatologist and APP salary concerns.
Read ArticleLow Dose Naltrexone for Fibromyalgia Pain - Use it or Lose it?
Medscape recently reviewed off-label use of low dose naltrexone (LDN) in fibromyalgia (FM) and found it is deemed effective by some clinicians and patients, but 1) there is no FDA indication for LDN use in FM and 2) the data supporting its use is limited.
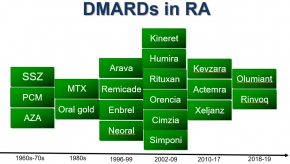
Read ArticleDMARD Treatment Lags for Minority Groups with Rheumatoid Arthritis
Use of disease-modifying anti-rheumatic drugs (DMARDs) for rheumatoid arthritis (RA) was markedly lower among all major racial-ethnic minority groups in the U.S. compared with white patients, analysis of federal survey data indicated, even after adjusting for income, education, and other factors.
Read ArticleChanging Loan Limits for Advance Practice Providers
The "One Big Beautiful Bill" (OBBB), or H.R. 1, was signed into law in July, calls for significant changes in the funding of healthcare education. This bill that lowers cap limits on education loans applies to graduate (APRN, PA) programs.
Read Article





Links: