All News
ANA Pollution (2.06.2026)
Dr. Jack Cush reviews the news, journal articles and regulatory news from this past week on RheumNow.com
Read ArticleGiant Cell Arteritis Outcomes in Canada
A retrospective cohort study of patients with giant cell arteritis (GCA) demonstrates relapses are common and seen in nearly half of patients, were common after treatment is stopped and is not effectively averted by methotrexate (MTX).
Read ArticleAortitis in Giant Cell Arteritis Treated with Tocilizumab
The The Tocilizumab in Giant Cell Arteritis Spanish Collaborative Group studied giant cell arteritis with aortitis, comparing the efficacy of intravenous vs. subcutaneously (SC) tocilizumab (TCZ) - demonstrating the superiority of SC TCZ.
Read ArticleThe 2025 Rheumatology Year in Review
The year 2025 presented numerous advances in rheumatology and related inflammatory and autoimmune disorders ranging from several new groundbreaking FDA approvals/indications, drug developments, game-changing guidelines and practices that will impact patient care for rheumatic diseases.
Read ArticleBest of 2025: Variability of Guidelines on the Perioperative Use of DMARDs in Rheumatic Diseases Patients
More effective treatments in patients with rheumatic diseases have resulted in less need for major surgery, yet a substantial number of RMD patients will undergo surgery often in the setting of DMARDs use. A scoping review looked at numerous clinical practice guidelines/recommendations for the perioperative management of DMARDs and found inconsistencies and low quality evidence, with great reliance on conditional (expert opinion) recommendations.
Read ArticleBest of 2025: ERS/EULAR guidelines for CTD-related interstitial lung disease
The European Respiratory Society (ERS) and European Alliance of Associations for Rheumatology (EULAR) have published clinical practice guidelines for the evaluation and management of connective tissue diseases (CTD) associated interstitial lung disease (ILD) in two simultaneous publications in the European Respiratory Journal and Annals of Rheumatic Diseases.
Read ArticleBest of 2025: Overview of the VEXAS Syndrome
A current systematic review in Rheumatology addresses the clinical features seen in the VEXAS syndrome.
Read ArticleDisease Activity Criteria for Adult-Onset Still's Disease: EULAR Points to Consider
EULAR has published a "points to consider" (PtCs) guidance on the development of criteria for the assessment of the disease activity in adult-onset Still’s disease (AOSD).
Read ArticleReefer Madness (12.12.2025)
Dr. Jack Cush reviews the news, journal and regulatory reports from this past week on RheumNow.com. B cell drugs in SLE and ITP, biomarkers in GCA & PSS and great videos by APPs.
Read ArticleTreating Refractory Still's Disease
A full read systematic review article examined treatment options for difficult to manage systemic juvenile idiopathic arthritis, or Still’s disease.
PET Uptake in Large Vessel GCA – Does tocilizumab help?
Giant cell arteritis (GCA) is the most common form of adult vasculitis in the United States and can be subdivided into those with cranial only symptoms, those with large vessel vasculiti/PMR, and those with mixed features.
Read Article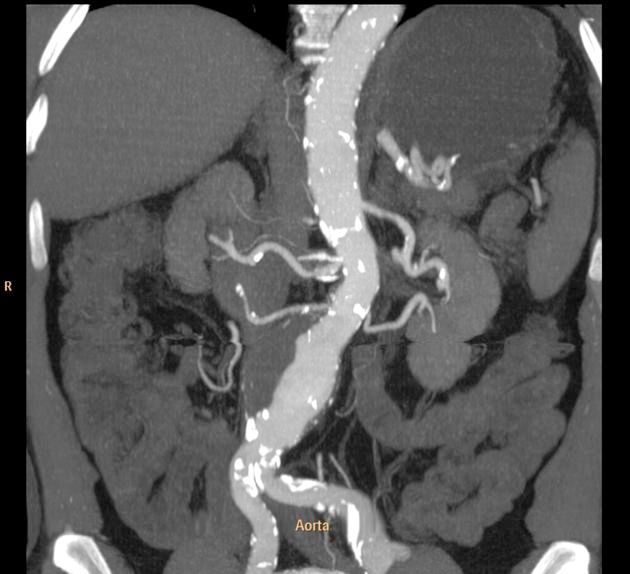

Links:




Mrinalini Dey DrMiniDey ( View Tweet)