Apremilast Succeeds in Scalp Psoriasis Save
Celgene has announced results from the phase 3 STYLE study, showing apremilast yielding significant improvement in moderate-to-severe scalp psoriasis.
Otezla is currently approved in the U.S. for active psoriatic arthritis and moderate-to-severe plaque psoriasis in adults.
STYLE is a phase 3, multicenter, randomized, placebo-controlled, double-blind study evaluating the efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis of the scalp. The study enrolled 303 people who were randomized 2:1 to receive apremilast 30 mg twice daily or placebo for the first 16 weeks.
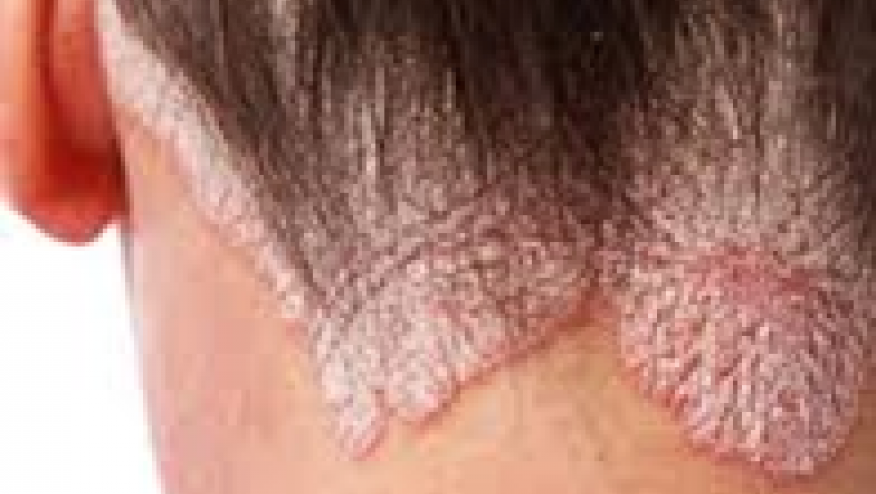
In this scalp psoriasis study, the primary endpoint was the Scalp Physician’s Global Assessment (ScPGA) response at week 16 compared with placebo. The scalp is one of the the most commonly affected and most difficult to treat areas in moderate-to-severe plaque psoriasis, and affects up to 80% of people with the skin disorder.
In addition to achieving the primary endpoint, statistical significance was also met for the secondary endpoint of the whole body itch as rated with the numeric rating scale.
No new safety signals were seen in this trial, with the most common features (apremilast vs PBO) being group diarrhea (30.5% vs 10.8%), nausea (21.5% vs 5.9%), headache (11.5% vs 4.9%) and vomiting (5.5% vs 2%).
Otezla is also in late-stage testing for Behçet’s disease, an inflammatory condition that affects many different parts of the body, such as the mouth, genitals and eyes. Other potential indications include rheumatoid arthritis and asthma.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.