Diffuse Cutaneous Systemic Sclerosis: Results of the SCOT Trial Save
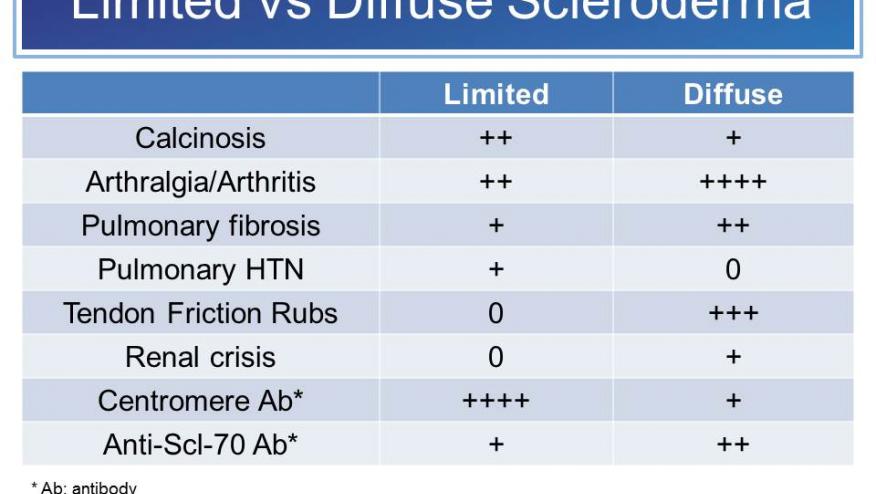
Diffuse cutaneous systemic sclerosis (dcSSc) is one of the most difficult autoimmune diseases to treat. In case of major organ involvement (lung, kidney), prognosis is poor and treatment options are limited.
The Cyclophosphamide Or Transplantation (SCOT) multicenter trial was designed to evaluate for long term benefit of myeloablative autologous HSCT over CYC.
75 patients with dcSSc and high risk of lung and/or renal involvement were randomized to CYC 750mg/m2/mo) or CD34+ selected autologous HSCT after myeloablation (800cGy total body irradiation with lung and kidney shielding, 120 mg/kg CYC and 90 mg/kg anti-thymocyte globulin).
Study results revealed 54 months post treatment event-free survival (EFS) of 79% in the HSCT group and 50% in the CYC group ( P= 0.021). Although not statistically significant, overall survival in HSCT was 91% and 77% in CYC (P=0.19). DMARD use by 54 weeks was significantly higher in the CYC arm 44% as opposed to the HSCT arm 9% (P=0.001).Treatment related mortality 3% HSCT vs 0% CYC), rate of cytopenias and Herpes zoster infection were more frequent in the HSCT arm.
In conclusion, improved survival and decreased use of DMARDS in both peri-transplant period and long term was observed in HSCT group. Overall the SCOT trial supports myeloablative HSCT for treatment of dcSSc.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.