Guselkumab Tops Adalimumab in Psoriasis Trial Save
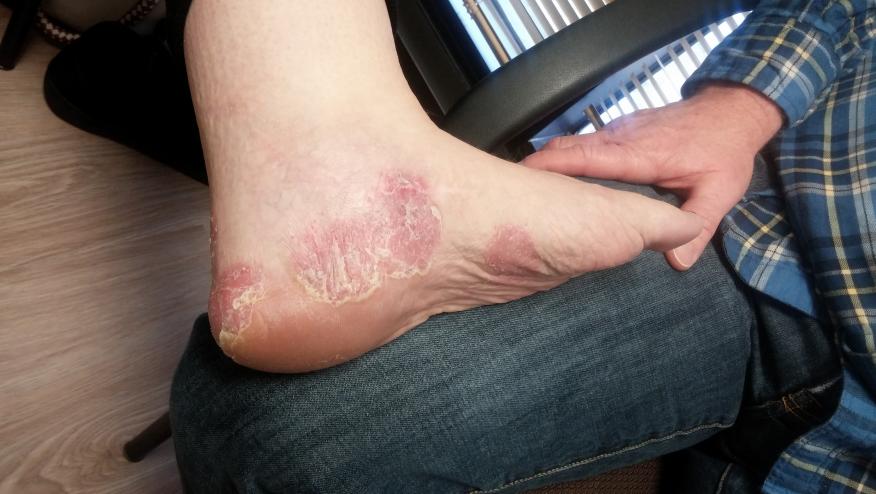
NEJM reports a 52 week, phase II trial in 293 adult patients with moderate-to-severe psoriasis (>10 BSA) who were randomized to receive either placebo, adalimumab or guselkumab - a novel IL-23 inhibitor.
A significantly higher proportion of patients in the guselkumab group had a score of 0 (cleared psoriasis) or 1 (minimal psoriasis) at weeks 16 and 40 compared to the adalimumab and placebo groups. At week 40, for example, 81 percent of patients taking a 200-mg dose of guselkumab had a score of 0 or 1, compared to 49 percent of patients taking adalimumab (Citation source http://buff.ly/1HOS2lS).
Guselkumab is a new and effective therapy for plaque psoriasis. Control of psoriasis may be achieved with specific anti–interleukin-23 therapy.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.