FOREMOST: Apremilast in Early Oligoarticular Psoriatic Arthritis Save
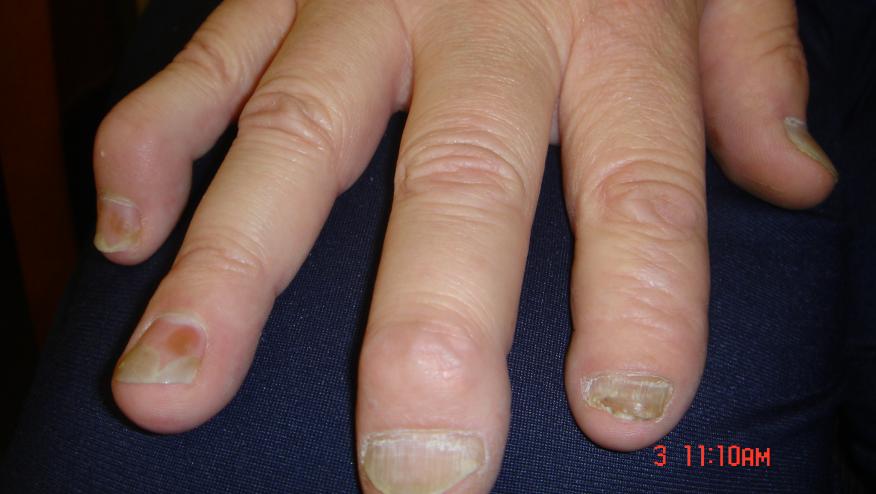
A controlled trial has shown that apremilast is effective in patients with early, oligoarticular psoriatic arthritis (PsA).
FOREMOST (NCT03747939) was a phase 4 multicentre, randomised, double-blind, placebo-controlled trial in early PsA (≤5 years) with oligoarticular involvement(>1 but ≤4 swollen and >1 but ≤4 tender joints; 2–8 total active joints). Patients were randomised (2:1) to apremilast 30 mg two times per day or placebo for 24 weeks. The primary endpoint was the achievement of minimal disease activity (MDA)-Joints (with ≤1 swollen joint and ≤1 tender joint) at week 16.
A tottal of 308 PsA patients (disease duration mean of 9.9 mos) were randomised and 40% of patients were taking a conventional synthetic disease-modifying antirheumatic drug.
The modified MDA response was significantly better with apremilast (33.9% and 21.3%) vs placebo (16.0% and 7.9%) at week 16 (p=0.0008 and nominal p=0.0028, respectively). Other patient-reported outcomes, clinical disease activity and skin measures also favored apremilast over placebo.
(Editors note: it is often said or assumed that apremilast is best suited for patients with early or milder PsA. But until the FOREMOST trial, there was no data to support this. The milder indication was based on milder results when apremilast was given to polyarticular, established PsA patients.)
The authors noted there has been limited data on drug effectiveness in oligoarticular PsA since most trials exclude patients with low counts of involved joints.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.