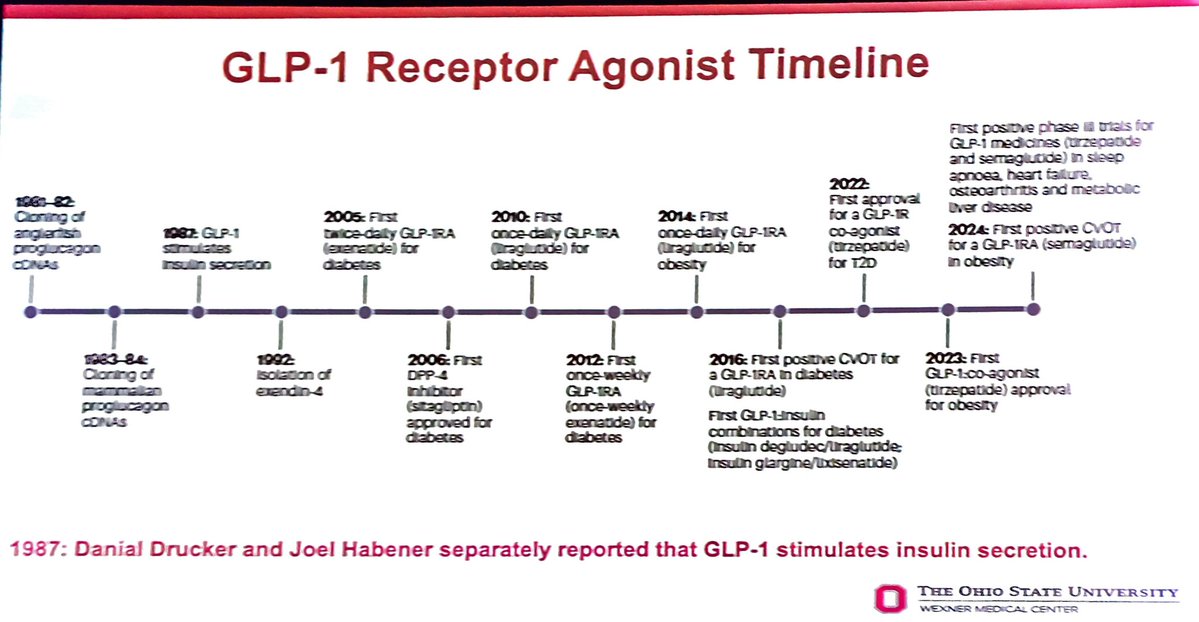
Early vs Delayed Belimumab in Lupus
An economic evaluation of early vs delayed use of the Blys inhibitor, belimumab (BEL), in systemic lupus erythematosus (SLE) has shown both cost effectiveness and clinical utility of early BEL initiation in active lupus patients.
While most would advocate for using your best therapy
Read Article