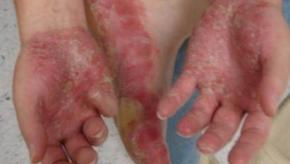
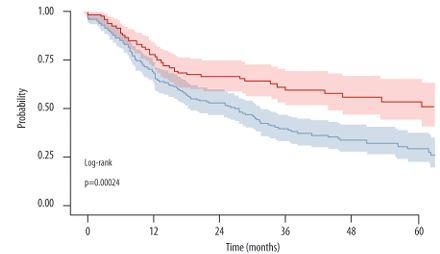
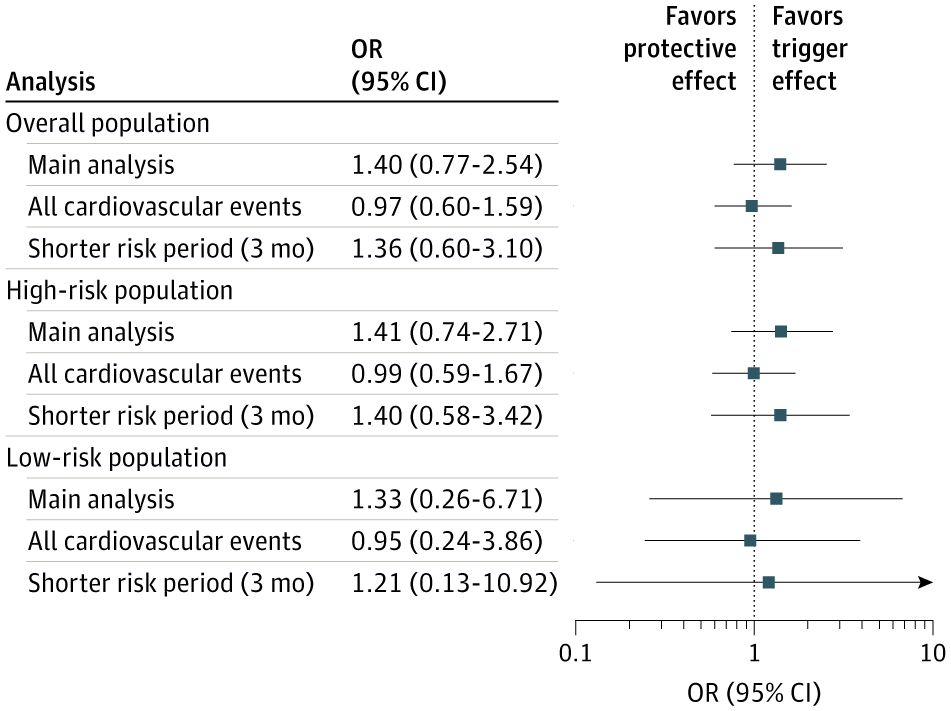
The 2025 Rheumatology Year in Review
The year 2025 presented numerous advances in rheumatology and related inflammatory and autoimmune disorders ranging from several new groundbreaking FDA approvals/indications, drug developments, game-changing guidelines and practices that will impact patient care for rheumatic diseases.
Read Article