3 Months of Romosozumab in Postmenopausal Osteoporosis
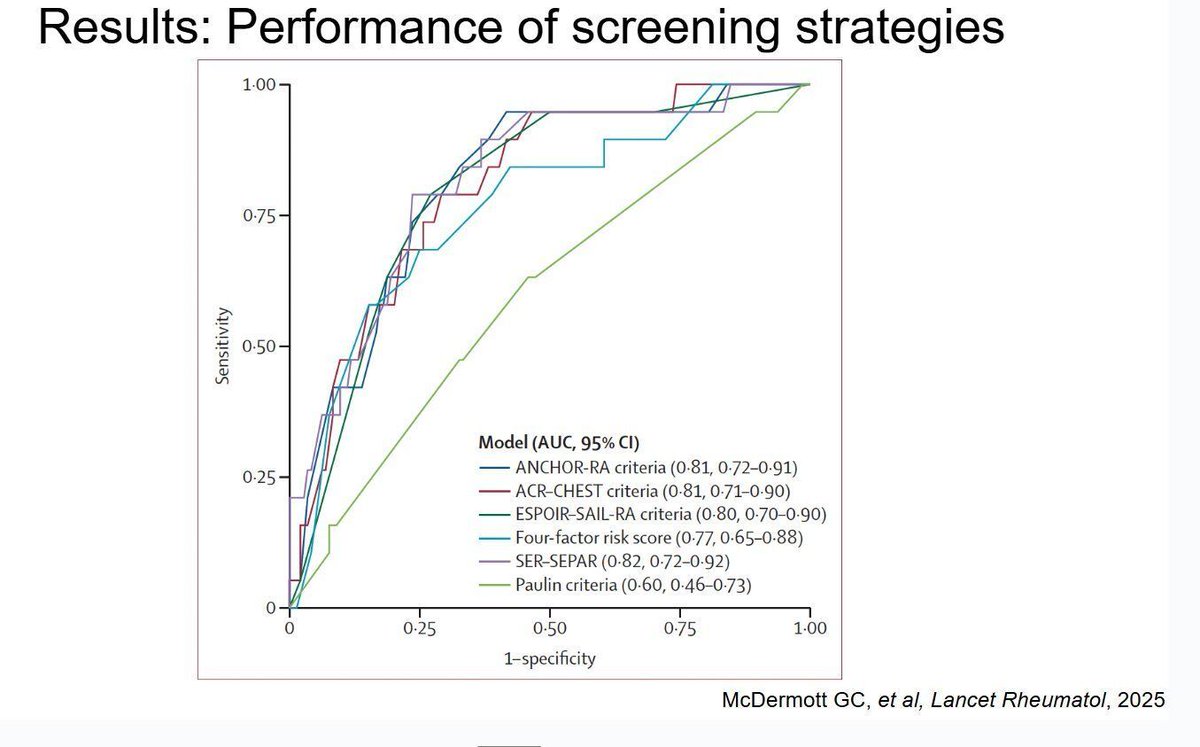
A report in The Lancet Diabetes & Endocrinology suggested that 3 months of romosozumab (ROMO) followed by denosumab is as effective at increasing hip bone mineral density (BMD) as the standard 12-month course.
Read Article