All News
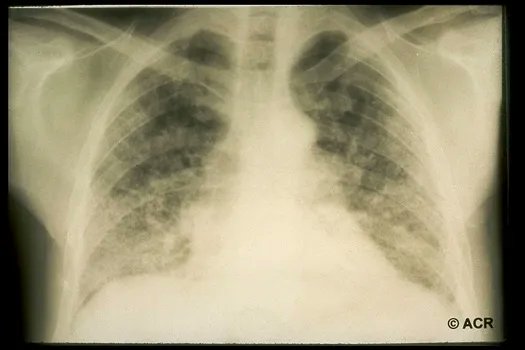
Best of 2022: Approach to ILD in Myositis Syndromes
Mehta et al have published a full read review of managing interstitial lung disease (ILD) in patients with inflammatory myopathies, a heterogeneous group of syndromes connected by ILD and and increased morbidity and mortality risk.
Read ArticleFever, FROST, & Pericarditis (12.16.2022)
Dr. Jack Frost reviews the news, and journal articles from the past week on RheumNow and ends with an audience question on "Macro-CK".
Read ArticleCOVID-19 Vaccine Responses in Rheumatic Disease Patients
Nature Reviews Rheumatology features a review of three notable papers that address the impact of SARS-CoV-2 vaccination on people with inflammatory rheumatic disease.
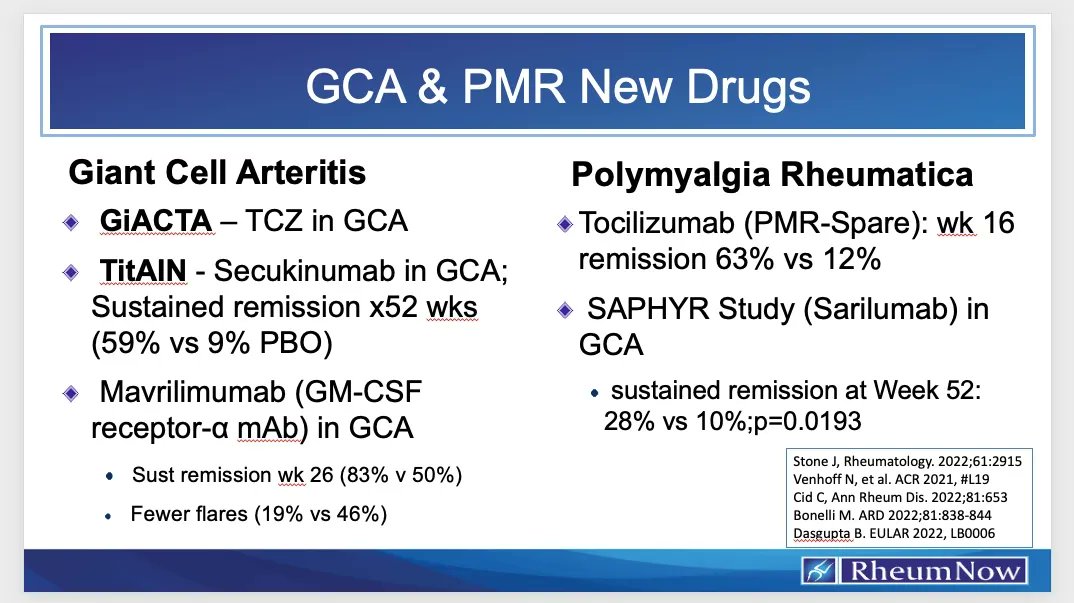
Read ArticleJAK Inhibitor Potential in Systemic Sclerosis-Associated ILD
The occurrence of interstitial lung disease (ILD) in Systemic sclerosis portends serious morbid and mortal outcomes for those affected. This review examines the available clinical literature on the potential benefits and outcomes of JAK inhibitor use in SSc=ILD.
Read Article
Links:

Links:
Links:

Links:

Links:

Review of the Rheumatic causes of Pericarditis and their management. Causes: SLE, RA, MCTD, Myositis, EGPA, GPA, IgG4-rel Dz, FMF, TRAPS, CAPS, AOSD, JIA, Sarcoidosis, Kawasakis, GCA, Takayasu's https://t.co/MQmkUe4Yey https://t.co/FSfrznoeBe
Links:
Links:

Links:

Links:

Links:

Links:

Links:
Links:

Links:
Links: