All News
RNL 2022: Spondyloarthritis Spectrum
This RheumNow Live 2022 session features highlights from lectures on Spondyloarthritis.
- Microbiome in Spondyloathritis - Jose Scher, MD
- Axial Psoriatic Arthritis - Philip Mease, MD
- Juvenile Spondyloathritis - Pamela Weiss, MD
Read Article
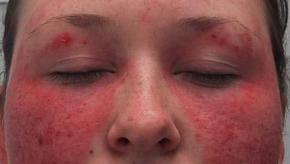
Rising Incidence of Systemic Lupus Erythematosus
Data from Olmstead county, MN over the past four decades shows that the incidence and prevalence of systemic lupus erythematosus (SLE) is increasing.
SLE was diagnosed using the EULAR/ACR criteria and looked at patients seen between 1976–2018.
Read ArticleMultimorbidity Burden in Rheumatoid Arthritis
Crowson and colleagues at the Mayo Clinic have shown that rheumatoid arthritis (RA) patients have a higher prevalence of multimorbidity with as many as 44 different morbidities of interest in RA.
Read ArticleAcute Inflammatory Responses Needed to Resolve Chronic Pain
Science Translational Medicine has published results of a Canadian study of low back pain (LBP) suggests that pain control by anti-inflammatory treatments might have negative effects on pain duration and may be counterproductive for long-term pain outcomes.
Read Article
Links:

Links:

Links:

Dr. John Cush RheumNow ( View Tweet)

Leonard Calabrese LCalabreseDO ( View Tweet)


Adam Cifu adamcifu ( View Tweet)
Links:

Rheum Cat rheum_cat ( View Tweet)


Links:

Links:
Links:

Links:

Links: