All News
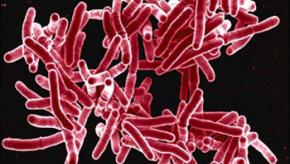
Low Risk of Latent TB with Secukinumab
TB reactivation and latent tuberculosis infection (LTBI) are warned against with biologic agents, especially TNF inhibitors; yet clinical trial data now shows that the risk of LTBI with IL-17 inhibition via secukinumab is uncommon.
Read ArticleSlow Lung Decline Typical in Systemic Sclerosis
The interstitial lung disease that develops in a subset of patients with systemic sclerosis tends to be heterogeneous, with the majority of patients experiencing a slow pattern of decline in lung function, analysis of outcomes in the European Scleroderma Trials and Research (EUSTAR) database foun
Read ArticleHydroxychloroquine Fails as Pre-exposure Prophylaxis for COVID-19
Numerous previous studies have questioned the efficacy or protection afforded by hydroxychloroquine (HCQ) for COVID-19 and studies have show that HCQ has failed when given as post-exposure prophylaxis to health care workers (HCWs). A new randomized controlled trial has shown that HCQ failed when given as 8 weeks of pre-exposure prophylaxis in hospital-based HCWs exposed to patients with COVID-19 patients.
Read ArticleNovel Biologic Promising in Mid-Stage Scleroderma Trial
The anti-interleukin agent romilkimab showed significant benefits on skin fibrosis among patients with systemic sclerosis in a phase II trial, researchers reported.
Read ArticleFilgotinib Approved by the EMA for Rheumatoid Arthritis
On Friday, Gilead and Galapagos announced that the European Commission (EC) has granted marketing authorization for filgotinib (trade name, Jyseleca) their oral, once-daily, JAK1 inhibitor for the treatment of adults with moderate to severe active rheumatoid arthritis (RA) who have resp
Read ArticleGabapentin Fails to Lessen Chronic Pelvic Pain
Lancet reports that in a randomized, blinded, controlled trial, gabapentin failed to significantly lower pain scores in women with chronic pelvic pain, and was associated with higher adverse event rates compared to placebo.
Read ArticleRheumNow Podcast – Cardiovascular Risk in Psoriatic Disease (9.25.20)
Dr. Jack Cush reviews the news and Journal articles from the past week on RheumNow.com.
Read ArticleEULAR Updated 2019 Recommendations for Psoriatic Arthritis Management
In June 2020, we failed to report on the ARD publication updating the 2019 update the European League Against Rheumatism (EULAR) recommendations for the pharmacological treatment of psoriatic arthritis (PsA) - these are presented below.
Read ArticleRomilkimab Effective in Early Diffuse Systemic Sclerosis
A phase II study of patients with early diffuse systemic sclerosis (SSc) has demonstrated improvement in SSc skin outcomes by targeting T cells and fibrosis with the bispecific monoclonal antibody romilkimab (RMK).
Read ArticleUstekinumab Early Risk for Cardiovascular Events
JAMA Dermatology reports a claims-based, case-time-controlled French study of 9290 psoriasis patients suggesting that ustekinumab initiation may be associated with an increased risk of acute coronary syndrome or stroke in high risk cardiovascular patients.
Read ArticleYoung Adults are Driving Up COVID-19 Rates
CDC reports that since June 2020 individuals infected with COVID-19 have largely been young adults in the 20-29 year age bracket. Moreover, younger adults appeared to be driving community transmission of COVID-19.
Read ArticleLivedo, Purpura and Coagulopathy in Severe COVID-19
JAMA Dermatology expands the spectrum of complications seen with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection describing cutaneous purpura and livedo lesions in patients with severe COVID-19.
Read ArticleCXCR3 and Restricted T Cells in Psoriatic Arthritis Inflammation
Research from Oxford University suggests a potential pathogenic role for the chemokine CXCR3 and clonally restricted CD8+ T cells in the pathogenesis of psoriatic arthritis (PsA) based studies of PsA synovial fluid samples obtained by arthrocentesis from 11 patients with large-joint oligo Ps
Read ArticleDMARDs for Early Rheumatoid Arthritis Have Cardiovascular Benefits
Patients with RA have up to a three-fold increased risk of mortality versus the general population, largely due to an increased incidence of premature CVD, such as atherosclerosis and heart failure.
Read ArticleRheumNow Podcast – Lupus Delights (9.18.20)
Dr Jack Cush reviews the news and journal reports from the past week on RheumNow.com:
Read ArticleCOVID-19 Risks in Pregnant Women
There has been little science or evidence regarding the effects of COVID-19 infection on pregnancy and vice versa; and now the recent MMWR addresses the subject with two new reports
Read Article
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)