BMS Tyk 2 Inhibitor Benefits Psoriasis Save
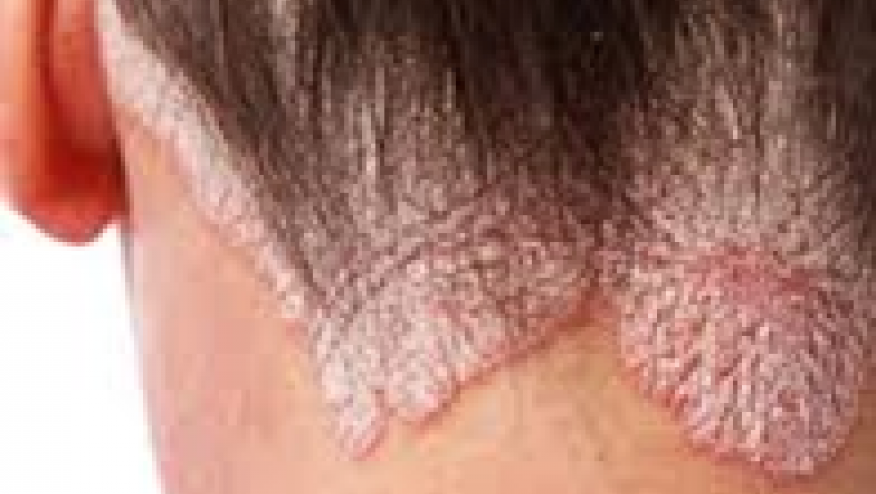
The NEJM reports that an oral selecive Tyrosine kinase 2 (TYK2) inhibitor of TYK2 was shown to be superior to placebo in a 12 week trial in patients with active psoriasis.
In a 12 week, phase 2, double-blind trial, BMS-986165 (TYK2 inhibitor) or placebo was given to 267 patients with active moderate-to-severe psoriasis, who previously failed to respond to other targeted cytokine inhibitors. Patients received wither 3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, or 12 mg daily or to receive placebo. The primary end point was a 75% or greater reduction from baseline in the Psoriasis Area and Severity Index (PASI75) score at week 12.
At week 12, the PASI 75 response rates were:
- PBO: 7% (3 of 45 patients)
- 3 mg of BMS-986165 qod; 9% (4 of 44 patients) (P=0.49 vs. placebo)
- 3 mg qd: 39% (17 of 44 patients) (P<0.001 vs. placebo)
- 3 mg bid: 69% (31 of 45 patients) (P<0.001 vs. placebo)
- 6 mg bid: 67% (30 of 45 patients) (P<0.001 vs. placebo)
- 12 mg qd: 75% (33 of 44 patients) (P<0.001 vs. placebo).
Discontinuation rates were low, generally about 2% (ranging from 2-7%) and there were 3 serious adverse events in patients receiving the active drug, as well as one case of malignant melanoma 96 days after the start of treatment.
Targeted inhibition of TYK2 with the oral agent BMS-986165 at doses of 3 mg daily and higher resulted in significant clearing of psoriasis.
(Funded by Bristol-Myers Squibb; ClinicalTrials.gov number, NCT02931838.)









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.