Intraarticular IL-1Ra Gene Therapy in Knee Osteoarthritis Save
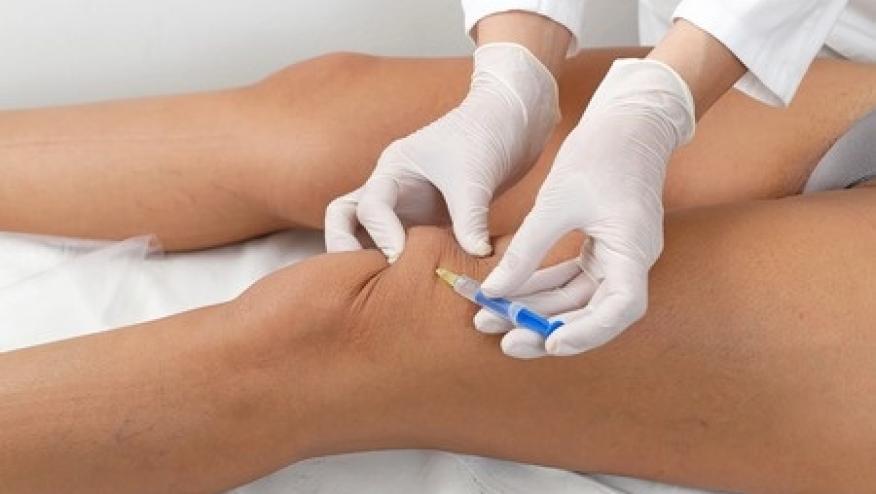
De la Vega et al. have published in Science Translational Medicine the efficacy of an IL-1Ra gene therapy in adults with knee osteoarthritis (OA).
In a dose-escalation trial in nine participants, they observed that a single intra-articular injection of the gene product led to an increase in IL-1Ra in the synovial fluid that remained elevated for up to a year. There were no serious adverse events related to the gene delivery.
This open-label clinical trial enrolled nine patients with radiographic knee OA. The IL-1Ra gene was delivered by a self-complementary (sc)–recombinant adeno-associated virus (rAAV) serotype 2.5 (sc-rAAV2.5IL-1Ra) by intra-articular injection into an index knee at one of three doses - low [1 × 1011 viral genomes (vg)], mid (1 × 1012 vg), or high (1 × 1013 vg). The primary outcome was safety.
Overall, there were no serious adverse events (AEs) related to sc-rAAV2.5IL-1Ra. Two AEs (knee effusions) occurred that were possibly related to the vector. The intervention (sc-rAAV2.5IL-1Ra) did not cause changes in vital signs, physical findings, or clinical laboratory measures.
Less than 1% of the injected dose of sc-rAAV2.5IL-1Ra vg was detected in circulation after 1 day and cleared within a week. Neutralizing antibodies to AAV2.5 were found in both the serum and synovial fluid.
More importantly, IL-1Ra concentration increased in the synovial fluid, and remained elevated after 1 year. During this period knee OA patient pain and function scores improved.
Intra-articular gene therapy with IL-1Ra was safe. The sustained intraarticular production of IL-1Ra, coupled with improved pain suggests the need for further clinical trials of such therapy in OA.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.