FDA REMS/Safety Panel Backs Educational Requirements for Opioid Prescribers
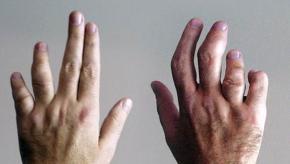
An advisory panel to the Food and Drug Administration met May 3rd and 4th to review whether the long-acting or extended release (ER/LA) opioid REMS programs approved in 2012 have had a meaningful effect on the opioid abuse problems noted nationwide. The FDA panel voted overwhelming