SATISFACTION (2.27.2026)
Dr. Jack Cush reviews the news and journal reports from this past week on RheumNow.com.
Read Article

Dr. Jack Cush reviews the news and journal reports from this past week on RheumNow.com.
Read Article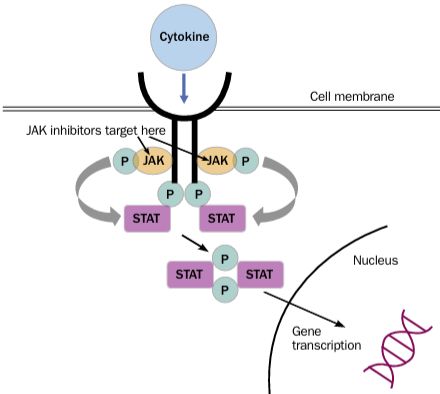
Dr. Jack Cush reviews the news and journal reports from this past week on RheumNow.com. Highlights include referral rules, combination biiologics in psoriasis and don't use JAK inhibitors in pregnancy.
Read ArticleGiant-cell arteritis and Takayasu’s arteritis are both large-vessel vasculitides, but each brings its own diagnostic and management challenges. Many of the difficulties overlap because they are rare, heterogeneous, and lack perfect diagnostic tests or specific biomarkers.
Read Article
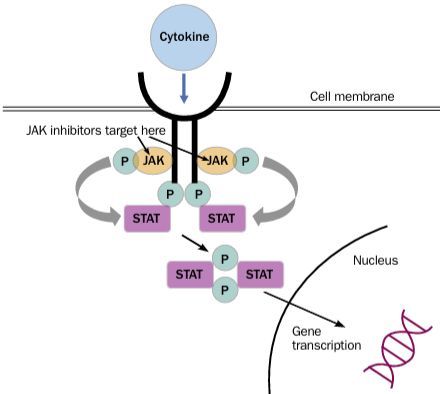
In 2020, the ACR Recommendations on Reproductive Health notably avoided firm guidance on the use of JAK inhibitors (JAKi) during pregnancy stating, "There is no available evidence regarding use or safety of the new small- molecule agents, tofacitinib, baricitinib, and apremilast, during
Read ArticleSunday’s vasculitis session at RNL26 was a fantastic update on inflamed blood vessels, large and small, by two experts in the field: rheumatologist Dr. Mike Putman and dermatopathologist Dr. Clay Cockerell.
Read ArticleDr. Jeffrey Sparks gave a state of the art update on Advances in RA-ILD, many of which he and his group have played a big part in, on Saturday at RNL26.
Read ArticleDr. Jack Cush reviews the news, journal articles and regulatory news from this past week on RheumNow.com
Read Article
A retrospective cohort study of patients with giant cell arteritis (GCA) demonstrates relapses are common and seen in nearly half of patients, were common after treatment is stopped and is not effectively averted by methotrexate (MTX).
Read Article
Dr. Jack Cush reviews the news and reports from the past two weeks on RheumNow.Com
Read ArticleAmong rheumatoid arthritis (RA) patients responding to upadacitinib (Rinvoq) or adalimumab, those receiving the former had significantly less residual pain with treatment, according to a post-hoc analysis of phase III trial data.
Read Article
The year 2025 presented numerous advances in rheumatology and related inflammatory and autoimmune disorders ranging from several new groundbreaking FDA approvals/indications, drug developments, game-changing guidelines and practices that will impact patient care for rheumatic diseases.
Read ArticleAdvanced practice clinicians (APCs; nurse practitioners and physician assistants) deliver a large share of US dermatologic care, accounting for 37% of clinicians and 27% of dermatology visits by 2020. A current JAMA Dermatology reports APC drug spending trends in dermatology, with a focus on
Read ArticleDr. Jack Cush reviews the news, announcements and journal articles from this past week on RheumNow.com. More on Variable bendability, a better way to treat RA, and a novel advance for GLP1a in PsA; and 2026 Resolutions!
Read ArticleEditor's note: This article was originally published April 22, 2025, and is being shared again as part of RheumNow's 'Best of 2025' series as we close out the year. Enjoy!
Read ArticleMore effective treatments in patients with rheumatic diseases have resulted in less need for major surgery, yet a substantial number of RMD patients will undergo surgery often in the setting of DMARDs use. A scoping review looked at numerous clinical practice guidelines/recommendations for the
Read Article



By downloading this material, I acknowledge that it may be used only for personal use and personal education and that I will accredit RheumNow.com as the source and owner of this material. Commercial use or mass reproduction of this material without permission from RheumNow (info@rheumnow.com) is prohibited.