All News
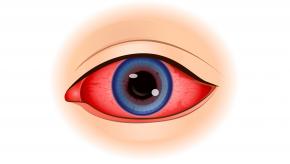
Putting IL17i into ‘focus’ for SpA-associated uveitis
The longest studied drug for uveitis in SpA has been monocolonal TNFi Abs. Reductions of acute anterior uveitis have been found with etanercept but less impressively than adalimumab, infliximab, and in my opinion less than golimumab and certolizumab pegol.
Other data have emerged for JAKi and IL17i, with respect to acute anterior uveitis.
Clinical and Therapeutic Challenges in Connective Tissue Disease and ILD
Connective tissue diseases (CTDs) and interstitial lung disease (ILD) represent a challenging intersection of systemic autoimmunity and progressive respiratory impairment. Research presented at EULAR 2025 continues to highlight the importance of CTD-ILD and the evolving landscape of therapeutic options for patients with autoimmune ILDs.
Read Article

Links:

Dr. John Cush RheumNow ( View Tweet)


Mrinalini Dey DrMiniDey ( View Tweet)

Mrinalini Dey DrMiniDey ( View Tweet)

Jiha Lee JihaRheum ( View Tweet)

Links:


Bella Mehta bella_mehta ( View Tweet)


Links:

Links:
Links:

Links:


Links: