ALTO: Long-Term Abatacept Outcomes in At-Risk RA Save
Lancet Rheumatology has published the ALTO results - long term outcomes of the APIPPRA trial, demonstrating that treatment with abatacept (ABA) in at risk patients can delay progression to rheumatoid arthritis (RA) for up to 4 years.
The Arthritis Prevention In the Preclinical Phase of Rheumatoid arthritis with Abatacept (APIPPRA) phase 2b, randomised controlled trial enrolled 213 anti-citrullinated protein antibody (ACPA) positive individuals with arthralgia. These individuals were randomized to received either placebo or weekly subcutaneous injections of 125 mg ABA for 52 weeks. Patients were followed for an additional 12 months off therapy to assess the protective potential of ABA. The The primary endpoint was having 3 swollen joints, meeting 2010 ACR/EULAR criteria for RA or starting DMARD therapy. The primary outcome was also stratified by autoantibody profiles at baseline.
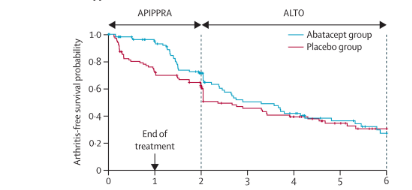
The APIPPRA Long-Term Outcome (ALTO) study extended follow-up for between 4 and 8 years with study participants and clinical assessors remaining blinded.
A total of 143 APIPPRA patients enrolled in ALTO (71 ABA; 72 PBO) with a median follow-up of 55 months. Primary events increased by 54 to 119.
The initial outcomes of APIPPRA (developing RA) were significant at 12 mos (6% ABA vs 29% PBO) and 24 mos (27% ABA vs 38% PBO). These remained significant in the ALTO extension out to 4 years (48% ABA vs 56% PBO), although the magnitude of treatment effect diminished over time.
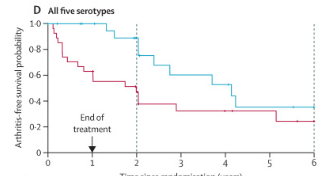
Patients with a broad RA autoantibody profile ("5 serotypes") at baseline were at highest risk of progressing, this subgroup showed the greatest abatacept benefits over time.
There were 18 serious adverse events in the abatacept group and 13 in the placebo group; none deemed related to study drug.
In this at-risk population, 1-year treatment with abatacept delayed progression to rheumatoid arthritis for up to 4 years. Those at highest risk of progression have a broad autoantibody profile but are more responsive to abatacept treatment.
ADD THE FIRST COMMENT
Disclosures
The author has no conflicts of interest to disclose related to this subject










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.