Biosimilar Slow Growth in USA Save
A recent Forbes article, "Biosimilars Are Making Inroads In U.S., But It’s Still A Bumpy Ride", reviews the current state and reasons for slowish uptake of these significant cost-savings alternatives to biologic therapy in treating cancer and autoimmune disorders.
The following are takeaway bullets from this informative article:
- Biosimilar entry into the market has been impeded by "including persistent and lengthy patent litigation battles and dynamics in the payer or pharmacy benefit manager space"
- The difference between biosimilar introduction in Europe vs USA is the patent disputes pervading the USA
- Enbrel biosimilars are widely available in Europe but not the USA. FDA approved in 1998, Two Enbrel biosimilars arose in 2016 and 2019, but will not be available in the USA until until 2029. Worldwide the Enbrel biosimilar market share has ranged from 14% to 74%.
- In Europe, after the introduction of rituximab biosimilars, sales of the originator (Rituxan) dropped by 50%.
- In the USA, patent reforms and legislations is needed to curtail the delays in biosimilar introductions into the US market.
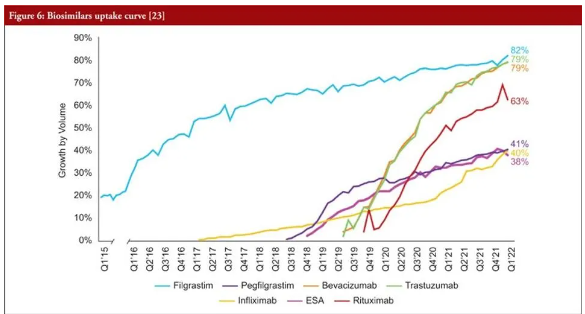
An Amgen 2022 report depicts the growth of heme-onc biosimilars,but also includes the growth of infliximab and rituximab biosimilars. This report does not depict the growth of newer adalimumab and ustekinumab biosimilars










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.