All News
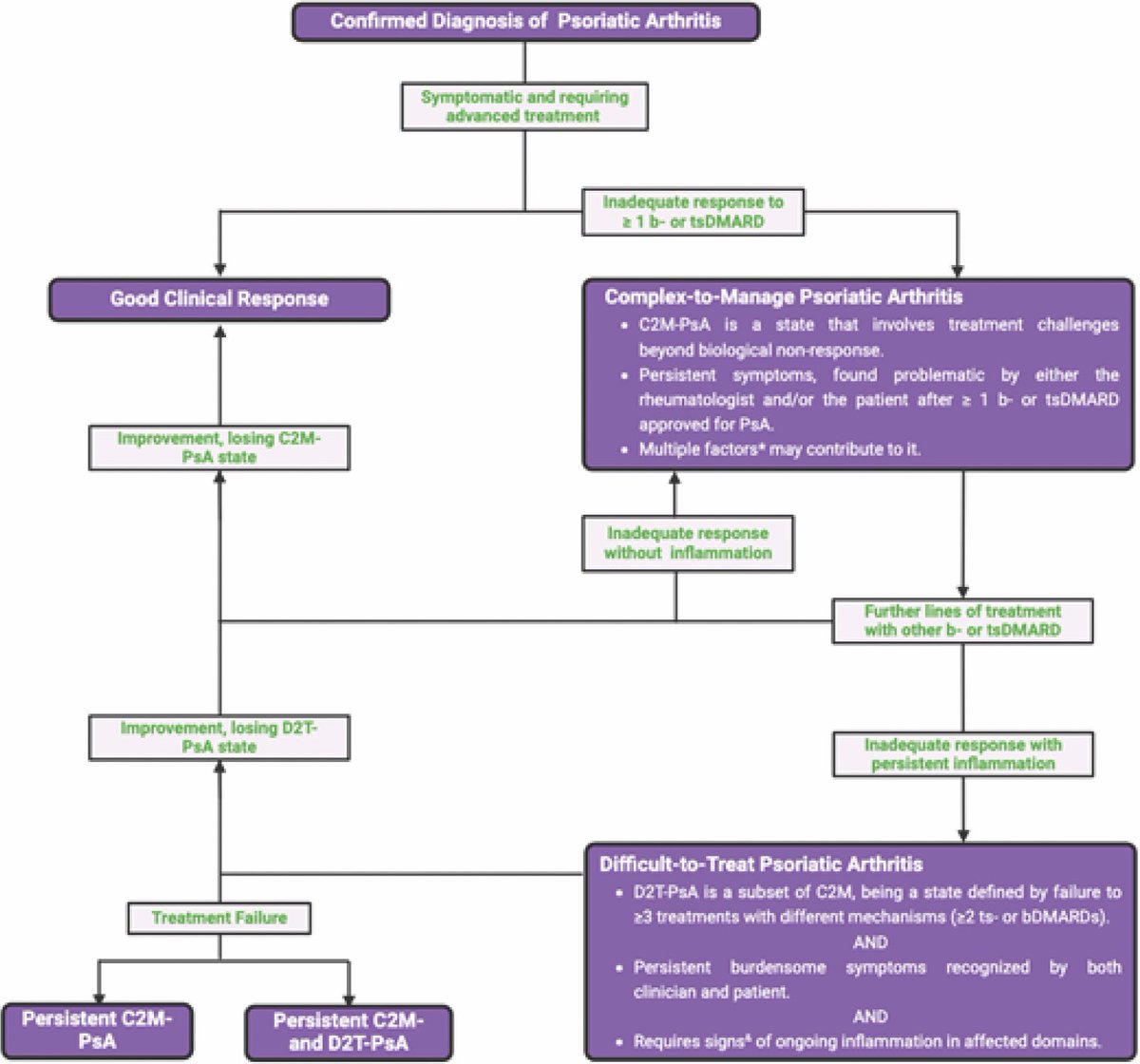
Difficult-to-treat & early PsA at #EULAR2025
GRAPPA definitions: D2T-PsA (≥3 therapies incl ≥2 b/tsDMARDs with persistent inflammation); C2M-PsA (broader: comorbidities, pain, intolerance). 95% GRAPPA consensus.
Abstact OP0175 @RheumNow #EULAR2025 https://t.co/eqKmMPh80x
Antoni Chan MD (Prof) synovialjoints ( View Tweet)

Leeds study of 223 ACPA+ Arthralgia pts w quant. MRI of hand showing tenosynovitis (34% at baseline) doubled the risk of progression to RA. Total tenosynovitis Volume = incr. RA risk #EULAR2025 POS0472 https://t.co/eT0wbhp1uN
Dr. John Cush RheumNow ( View Tweet)

▶️Erosive hand OA
🔺Gull-wing erosion @ DIPJs ✅
🔺Saw-tooth erosions @ PIPJs ✅
#OA #Osteoarthritis #EULAR2025 https://t.co/5Hh4Ki9P8V
Dr Gurdeep S Dulay gurdeep_dulay ( View Tweet)

Fast track PMR clinics in Denmark led to more patients seen, and time to referral went down.
If we don’t get in early with a right diagnosis (and maybe early steroid-sparing) then PMR patients deteriorate.
We shouldn’t let PMR be an end stage disease #EULAR2025 POS0136 @RheumNow https://t.co/pqwveamhJP
David Liew drdavidliew ( View Tweet)
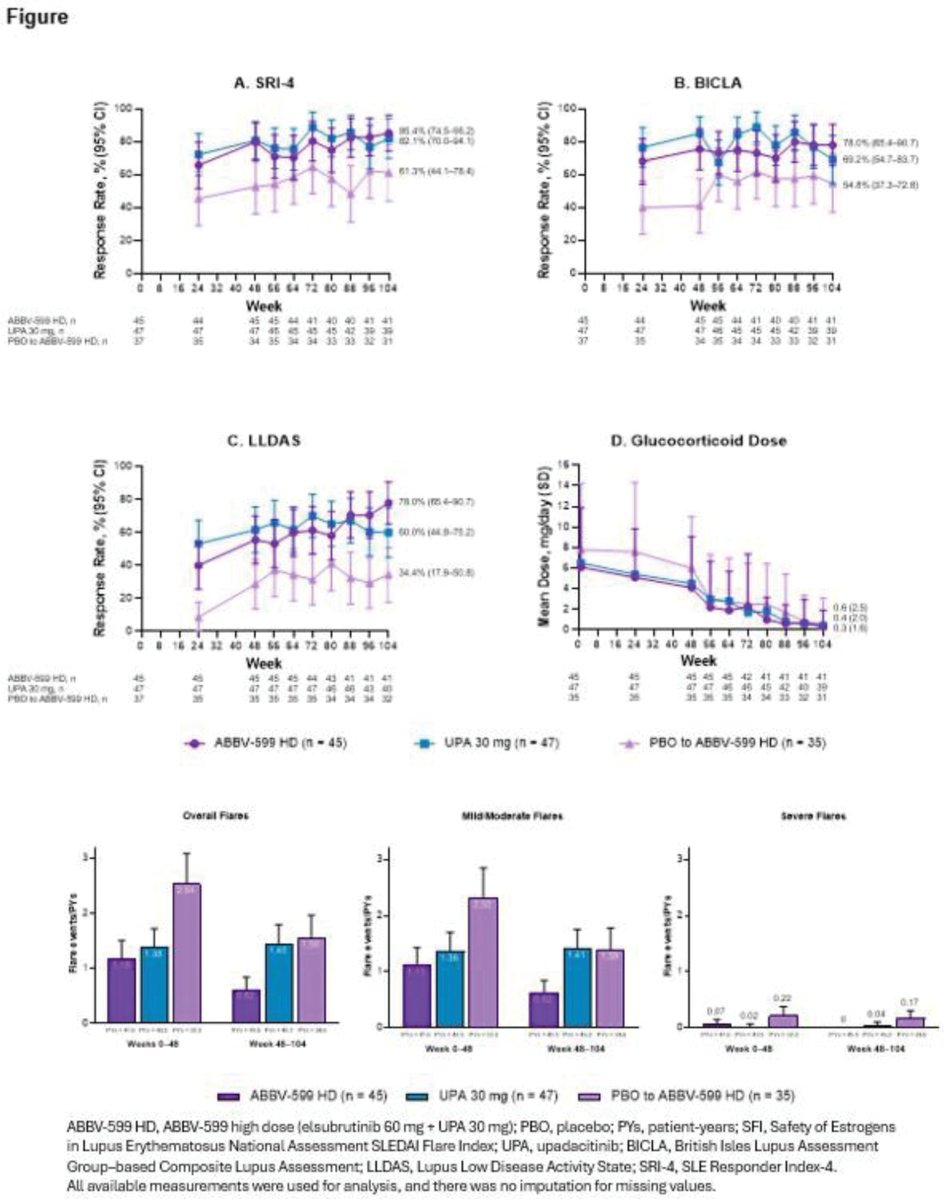
In SLEek LTE, ABBV-599 HD (BTKi+JAKi) & UPA 30mg sustained or improved disease control through 104wks: SRI-4 ≥82%, ↓flares, near steroid-free, no new safety signals. PBO-switchers improved too. Targeted oral combos look promising.
@RheumNow #EULAR2025 #OP0198 https://t.co/IHXC2iD0h6
Mrinalini Dey DrMiniDey ( View Tweet)

Data from Dutch registry SpA-Net explored prevalence using new definitions for D2M axSpA.
-Smoking and psoriasis were characteristics associated with D2M disease.
-In the three variations explored, the lowest prevalence was observed in the variation based purely on objective https://t.co/h3f4ORQl0F
Links:
Adela Castro AdelaCastro222 ( View Tweet)

Sex differences in biologic response in PsA.
DISCOVER-2 post hoc analysis showed:
•Radiographic progression at Week
♂️: 2.22 units
♀️: 1.10 units
•Early ACR-like response (cDAPSA LDA at 8wks) linked to less damage in men (0.39 vs 2.24)
Women less likely to progress https://t.co/NhBkXbuLjO
Antoni Chan MD (Prof) synovialjoints ( View Tweet)
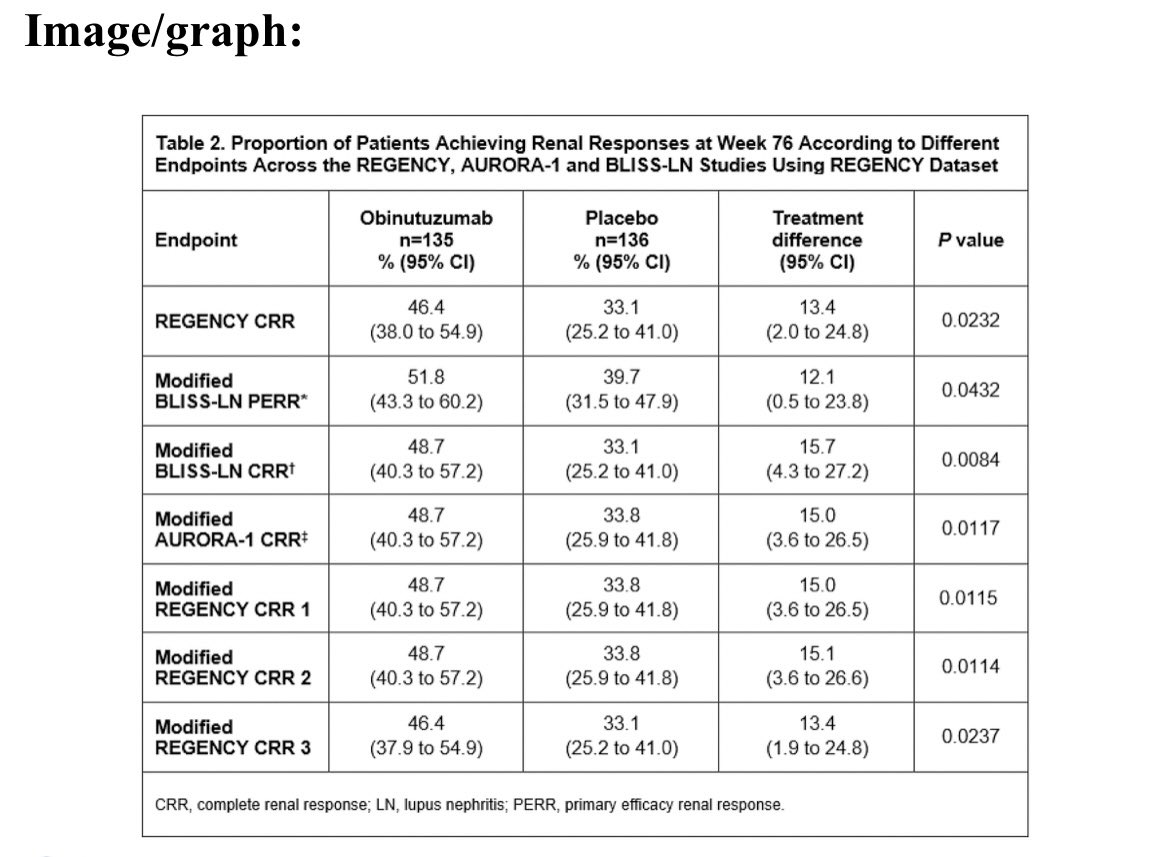
#EULAR2025 Abstr#OP0006. There is no standardised definition for complete renal response in #lupus nephritis. Post-hoc analysis of REGENCY showed Obinutuzumab + SOC was superior to PBO + SOC if endpoints for voclosporin and belimumab RCTs were used. Effect size ~13-16% @RheumNow https://t.co/q8MNAV8XF3
Md Yuzaiful Md Yusof Yuz6Yusof ( View Tweet)
Secukinumab Use in Refractory Giant Cell Arteritis
In 2023, the phase 2 TitAIN study showed that the effectiveness and safety of secukinumab in 52 patients with giant cell arteritis (GCA) who had an inadequate response to tocilizumab. While we await the results of a larger https://t.co/pG3aN3o4GZ
Dr. John Cush RheumNow ( View Tweet)

T2T in #gout w serum #uric #acid / #urate target
SAVES LIVES
Study of achievement of target 🎯
⬇️CVE and death
If not achieving Urate <360
T2T also reduced baseline #SUA
#EULAR2025 @rheumnow abst#OP0005 https://t.co/ePchJ4vqnU
Janet Pope Janetbirdope ( View Tweet)
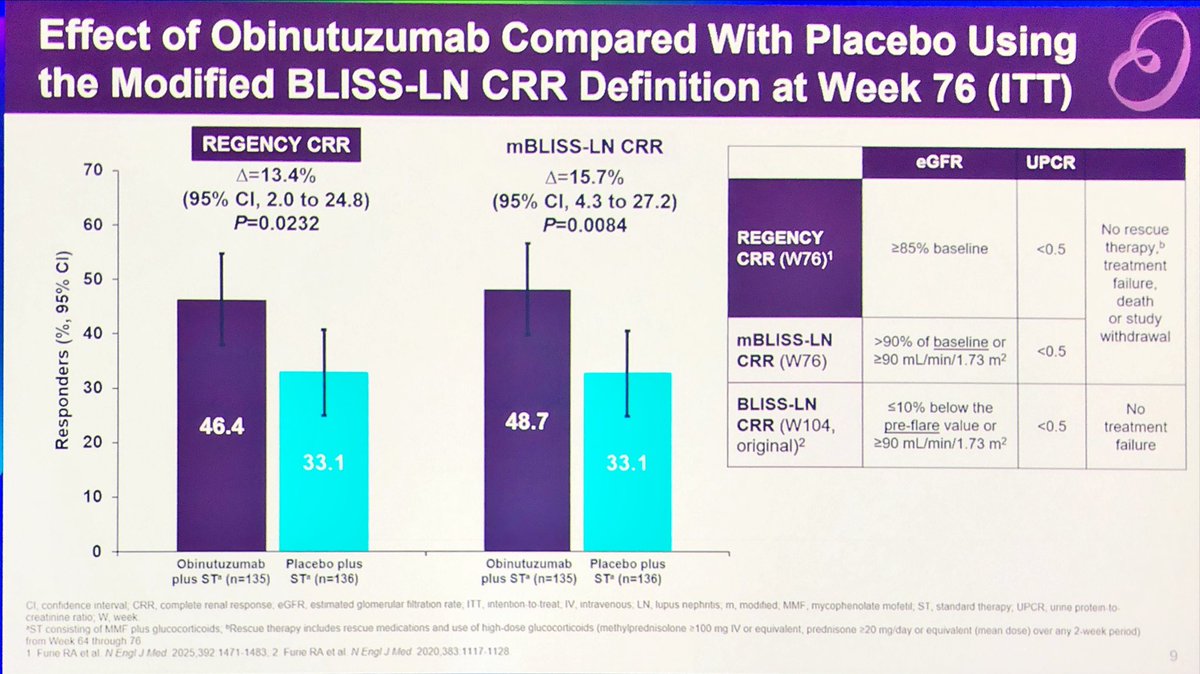
#Oninutuzumab works in
#lupus #nephritis #LN
no matter how you vary the
#renal #responses
Of various definitions of
#Complete & #Partial #renal #responses
abst#OP0006
#EULAR25 @eular_org @RheumNow
Not surprised 😲 https://t.co/Zn2lXkddez
Links:
Janet Pope Janetbirdope ( View Tweet)

1 shot will do!
#symptomatic #Knee #OA given #gene #therapy #intra-#articular
Seemed to last up to 104 weeks!
But no placebo
small safety study but sustained #WOMAC responses
Needs large #RCT but v interesting
#EULAR2025 @RheumNow @eular_org
Abst# POS0492 https://t.co/hqTqBorrbA
Links:
Janet Pope Janetbirdope ( View Tweet)

Exciting results from ARGO trial:
-Phase 2 of Sonelokimab (dual IL-17A-IL17F) nanobody in PsA.
-Met primary endpoint of ACR50 at week 12 vs PBO.
-62% achieved MDA
-48% achieved composite of ACR 70+PASI 100
Looking forward to phase 3 results!!!
Abstract #OP0096 #EULAR2025 https://t.co/1D52eG8ReS
Links:
Adela Castro AdelaCastro222 ( View Tweet)

Insights from the Gut-Joint Axis in axSpA: Findings from the DESIR cohort
-Baseline biomarkers of bacterial translocation: PLTP (phospholipid transfer protein) and LPS evaluated.
-In early axSpA baseline PLTP activity was associated with sacroiliac radiographic progression at 5
Adela Castro AdelaCastro222 ( View Tweet)

So much needed in SLE and pregnancy
1. Risk stratification at diagnosis
2. Pregnancy counseling at dx and >1 y before pregnancy
3. Close collab with GYN necessary
4. Challenging management given gaps in evidence.
#EULAR2025 #asktheexpert @RheumNow https://t.co/MXGkKpl452
Links:
Adela Castro AdelaCastro222 ( View Tweet)

Secukinumab for PMR?
-post hoc analysis of the TitAIN study (phase 2 RCT on new onset/relapsing GCA) showed:
-Numerical reduction in patients experiencing PMR symptoms when treated with secukinumab compared to placebo.
-Safety profile was similar to the overall GCA study
Adela Castro AdelaCastro222 ( View Tweet)

Biomarker data suggest TNFi non-responders in PsA exhibit upregulation of IL-17F gene signatures after treatment failure. Supports IL-17A/F blockade rationale with bimekizumab in TNFi-experienced patients Abstract#OP0091 @RheumNow . #EULAR2025 https://t.co/CWR6ArDRRW
Antoni Chan MD (Prof) synovialjoints ( View Tweet)

Real-world study on dual bDMARD plus JAKi or TYK2i combinations in refractory PsA. 22 PsA patients on dual bDMARD + JAKi/TYK2i:
•Most common: IL-17i + TYK2i
•Total exposure: 8.5–10.5 pt-years
•Only mild URIs/stomatitis
•Clinical improvements seen in joint/skin domains https://t.co/waDReDqSDE
Antoni Chan MD (Prof) synovialjoints ( View Tweet)

SPEED RCT: In early PsA with poor prognostic factors, PASDAS at 24wks:
•Early TNFi: 3.7
•Combo csDMARDs: 4.1
•Step-up csDMARDs: 4.7
Early TNFi beat step-up by -1.09 (p<0.001); combo csDMARDs also superior (-0.69, p=0.02). Early TNFi benefit sustained at 48wks. Abstract#OP0089 https://t.co/MpazF8BIr2
Antoni Chan MD (Prof) synovialjoints ( View Tweet)

Precision immunotherapy in axial spondyloarthritis: TRBV9xCD3 bispecific antibodies selectively depleted autoreactive TRBV9+ T cells from HLA-B27+ AS patient samples while sparing over 95 percent of the T cell repertoire. TRBV9xCD3 bispecific antibodies selectively depleted https://t.co/8UXqEaDYnS
Antoni Chan MD (Prof) synovialjoints ( View Tweet)



