2018 Swedish Guidelines for Giant Cell Arteritis Treatment Save
The Swedish Society of Rheumatology has developed evidence-based guidelines for the management of giant cell arteritis (GCA) with a focus on the appropriate use of corticosteroids and tocilizumab.
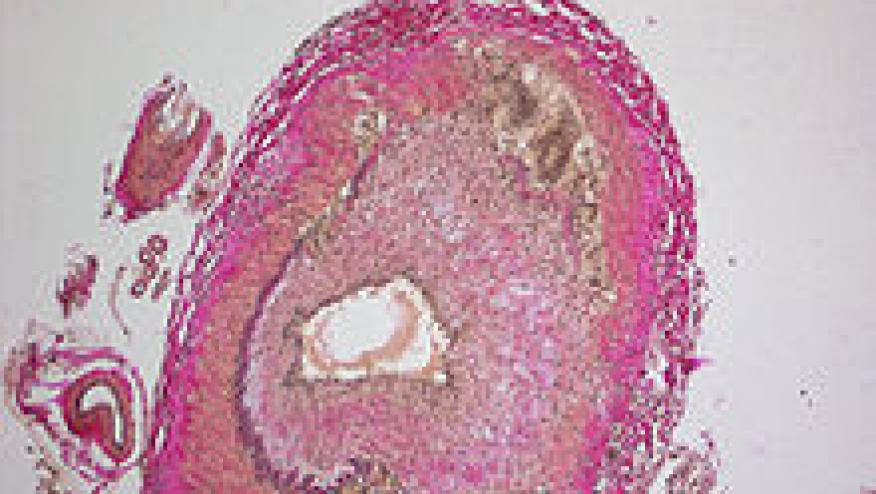
Beyond pharmacological treatment, they address the use of temporal artery biopsy (TAB) and other imaging techniques (magnetic resonance imaging and positron emission tomography/computed tomography) for the evaluation of large-vessel involvement.
Steroids (oral, or intravenous in cases with ischaemic complications) remain the first line for GCA, and tocilizumab (TCZ) is only recommended for patients with relapsing disease who meet one of five criteria for active disease. Current evidence suggests that tocilizumab treatment for > 1 year cannot be recommended.
Exerpts from this comprehensive guideline are provided below.
New Proposed GCA Classification
| Original criteria, ACR 1990 | Proposal for new, extended criteria* |
|---|---|
| Age at onset of disease ≥ 50 years | Age at onset of disease ≥ 50 years |
| New headache or new type of localized pain in the head, visual symptoms, loss of sight, PMR, constitutional symptoms, and/or jaw claudication | Any of the following: new headache of new type and localization, chewing, and/or jaw claudication |
| Tenderness at palpation or low pulse at the temporal artery | Tenderness at palpation or low pulse at the temporal artery and/or extracranial arteries |
| ESR ≥ 50 mm/h | ESR ≥ 50 mm/h and/or CRP ≥ 10 mg/L |
| Positive temporal artery biopsy (granulomatous arteritis or arteritis with mononuclear cells, giant cells) | Positive temporal artery biopsy (granulomatous arteritis or arteritis with mononuclear cells, giant cells) and/or imaging diagnostics using ultrasonography findings, MRI, and/or FDG-PET. |
*These criteria are based on opinions and should not be used in clinical practice or in clinical studies. A patient has GCA if three out of five criteria are met, provided the biopsy is positive and/or the imaging diagnostics results are compatible with a GCA diagnosis.
Recommendations for Glucocorticoid Use
- Glucocorticosteroids remain first line for the treatment of GCA.
- It is vital not to delay treatment, for example while waiting for a temporal artery biopsy.
- The recommended initial dose of prednisolone is 40–60 mg for 4 weeks, thereafter gradually tapered.
- If vision is impaired or there are other signs of serious vascular involvement, intravenous methylprednisolone 1000 mg once daily for 3 days may be considered, followed by oral treatment as above.
- The rationale for treating GCA with tocilizumab is primarily its glucocorticoid-sparing effect over time.
Suggested Schedule for Reducing Glucocorticosteroid Treatment for GCA
- 40–60 mg prednisolone/day for approximately 4 weeks (until ESR and CRP have been normalized, and signs and symptoms have improved
- Thereafter, reduction of the dose by 10 mg every other week to 20 mg daily
- Thereafter, reductions of 2.5 mg with 2–4 week intervals to 10 mg daily
- If there are no signs of relapse, the dose may be reduced by 1 mg every month or every other month.
- Follow-up and treatment modification After every dose reduction, the patient’s ESR and CRP are checked and the return of signs and symptoms is also checked.
- If signs and symptoms of active disease return, the dose of prednisolone should be increased to the latest effective dose.
Recommendations for Tocilizumab Use
- Tocilizumab is recommended as a supplement to prednisolone treatment in patients with recurrent or active illness during glucocorticoid treatment, providing the criteria set in the guidelines are met.
- In cases of newly diagnosed GCA, tocilizumab may be considered when there is a great risk of future side effects of glucocorticoids, and pronounced clinical and laboratory signs of vascular inflammation.
- Treatment with tocilizumab should be discontinued after 1 year. (Longer periods of treatment cannot be recommended with our present state of knowledge)
- If inflammation persists after 1 year of treatment with tocilizumab, an individual assessment must be made by the treating physician.
Criteria for Addition of Tocilizumab
- Relapse during glucocorticoid treatment or relapse after the completion of treatment with glucocorticoids
- Large vessel arteritis that has at some point been verified with biopsy or with imaging of large vessels (MRI, PET-CT, or CT angiography)
- Clinically active giant cell arteritis
- Elevated CRP and ESR
- Obvious side effects of glucocorticoid treatment or great risk of such side effects from future treatment with glucocorticoids
These draft guidelines were written by a small group of experts, based on current standards in Sweden, and with broad discussion within the rheumatology community.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.