EULAR: Tofacitinib Effective in Ankylosing Spondylitis Save
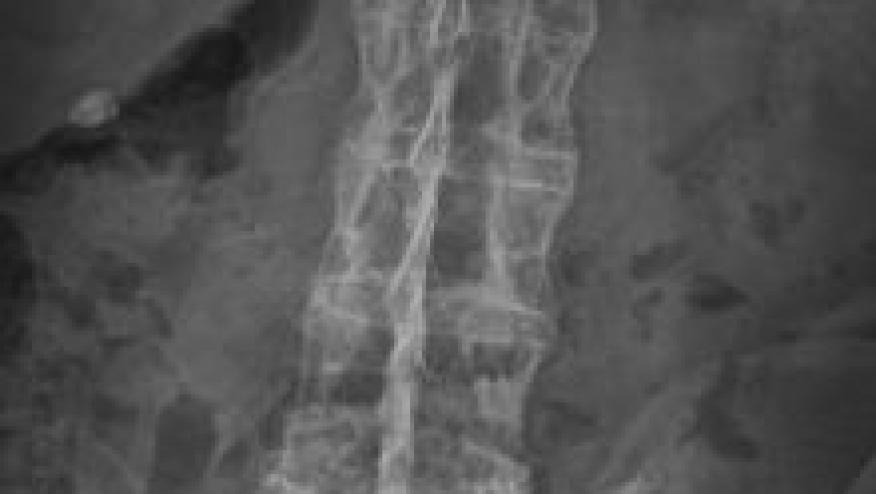
Desiree van der Heidje, MD, PhD presented the results of a 16-week, phase II, dose-ranging study of tofacitinib (TOFA) in patients with active ankylosing spondylitis. Both tofacitinib 5 and 10 mg BID demonstrated greater clinical efficacy compared with placebo as measured by ASDAS20 and ASDAS40 outcomes. Additionally, MRI imaging showed structural/inflammatory benefits in TOFA, but not placebo treated patients.
208 AS patients were randomised 1:1:1:1 to placebo or tofacitinib 2, 5 or 10 mg BID for 12 weeks plus 4 weeks follow-up. There were 196 completers, and 87% were HLA-B27 positive and 81% were white. The mean disease duration was 6.3 years and the mean baseline BASDAI was 6.7.
Treatment with TOFA (5 mg or 10 mg bid) yielded better ASDAS20 scores that were 23% adn 27% better than placebo rates (~40%). BASDAI results were similar. The ineffective 2 mg bid dose of TOFA had results comparable to placebo. No new safety signals were raised by this trial.
MRI changes were assessed by SPARCC scoring of the sacroiliac joints and spine. Overall, the 5mg and 10 mg TOFA group yielded significantly less change and inflammation in the spine.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.