Immunophysiology of the Gout Attack Save
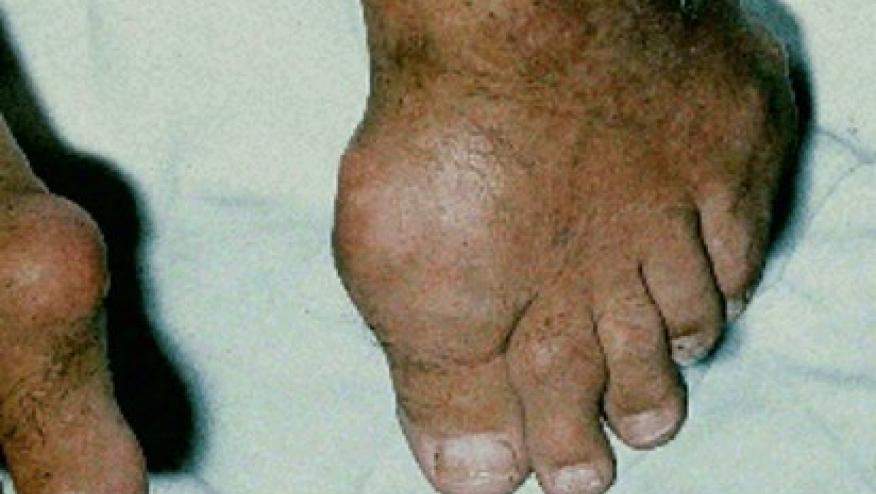
Gout may be an ancient disease, with arthritis of the big toe having been described in Egypt in 2,600 BC, but only now are the underlying pathophysiologic events being elucidated and understood.
Current thinking on the pathogenesis of gout, which today affects more than 8 million Americans -- 4% of the population -- has evolved into the "mobilization flare" hypothesis. In this disease conceptualization, when serum urate levels exceed 6 mg/dL, even in asymptomatic individuals, deposition of urate crystals in the synovium and other tissues begins.
"Gout represents the most hyperinflamed situation we know about," said Peter Lipsky, MD, from Charlottesville, Virginia, at the Florida Society of Rheumatology annual meeting.
Crystals of monosodium urate form in close proximity to each other and proteins and rapidly become an insoluble crystalline lattice structure, explained Lipsky, the former scientific director of NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases in Bethesda, Maryland.
The surface of that structure is quite active and is in equilibrium with uric acid levels in surrounding tissues. But with sudden changes in the microenvironment, which could be the level of uric acid, pH, temperature, or changes in proteins that increase their solubility, the lattice matrix becomes unstable and crystals begin to shed off the surface into the surrounding tissue, he noted.
"If the shedding is relatively modest, with only a few crystals being released, those can be opsonized with a variety of positively charged proteins including ApoE and they do not become inflammatory," he said.
However, if a large mass of crystals is shed, the crystals can begin to interact with receptors on immune cells, the process of inflammation begins, and if the crystals are opsonized with immunoglobulin, the process is further heightened.
"It's a very dynamic process occurring at the surface of this crystalline lattice structure, and it's the additional proteins in the environment that seem to control whether or not the crystals are shed," he noted.
Further understanding of the underlying processing leading to gout derived from work initially done at the NIH among children with autoinflammatory diseases, in which a cellular structure known as the inflammasome was described, according to Lipsky.
The NLRP3 inflammasome is capable of sensing the presence of a variety of intracellular molecules and can lead to the induction of inflammatory responses. When the molecule is the monosodium urate crystal and not soluble monosodium urate, the multiprotein structure assembles and releases activated caspase-1, which is the critical enzyme involved in the processing of interleukins (IL) 1 and 18 and other proinflammatory molecules. This leads to the production and release from the cell of active IL-1 and IL-18 -- and the inflammatory response.
"This is the basis of how we now understand that urate crystals stimulate inflammation, and is also the basis of why the IL-1 blocker canakinumab [Ilaris] seems to be so effective in controlling inflammation in gout," he concluded.
Dr. Lipsky disclosed a relevant relationships with Horizon Pharma.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.