Secukinumab Better than Adalimumab - Maybe? Save
The EXCEED study was a head-to-head trial of secukinumab (SEC) versus adalimumab (ADA) as first-line monotherapy in psoriatic arthritis (PsA) patients; this 52 week trial showed that while SEC failed to achieve clinical superiority over ADA, SEC treated patients demonstrated higher treatment retention rates and better PASI90 response rates.
Lancet reported the results of this multicenter trial enrolled 853 adult patients with active PsA and given blinded standard doses as monotherapy. The primary endpoint was superiority of SEC over ADA as measured by ACR20 response at week 52.
A total of 709 (83%) patients completed the trial. ACR20 responses at week 52 were similar - 67% for SEC group versus 62% for the ADA group (OR 1·30, 95% CI 0·98-1·72; p=0·0719). ACR50 (45% vs 49%) and change in HAQ (-.58 vs -.56) were also not different between groups. Yet the skin responses clearly favored SEC (PASI90 67% vs. 43%) over ADA.
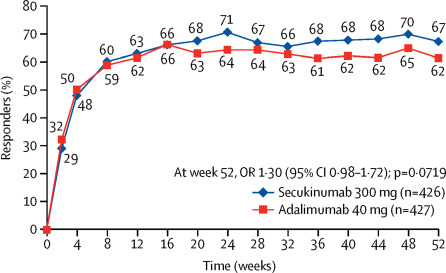
Discontinuations were higher in ADA (24%) compared to SEC (14%) treated patients.
The safety outcomes were as expected for both agents; with serious infections occuring in 2% of SEC patients and 1% of ADA patients. There was 1 death due to colon cancer (SEC).
Secukinumab did not meet statistical significance for superiority versus adalimumab in the primary endpoint of ACR20 response at week 52. However, secukinumab was associated with a higher treatment retention rate than adalimumab. This study provides comparative data on two biological agents with different mechanisms of action, which could help guide clinical decision making in the management of patients with psoriatic arthritis.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.