TNF Inhibitor Induced Psoriasis Save
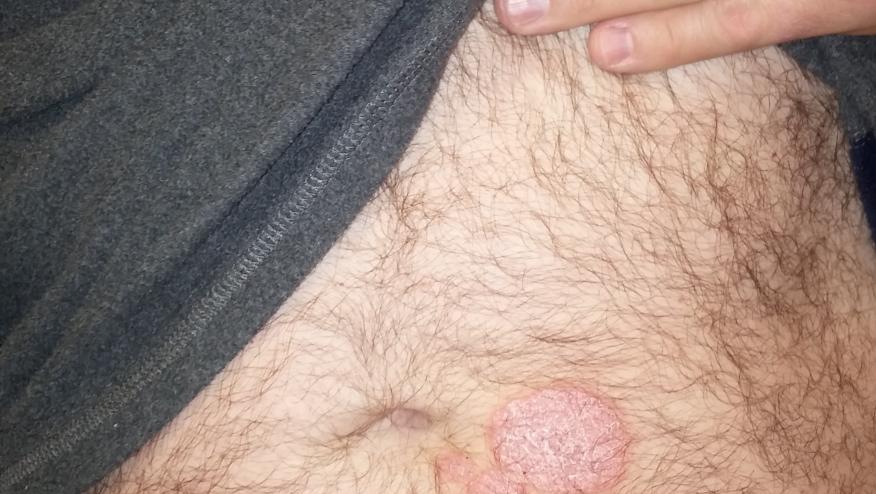
Tumor necrosis factor-α inhibitors (TNFi) rarely have been reported to induce new-onset psoriasis. Although the rate of this adverse event is estimated to be 1/1000, it occurs with all TNFi and occurs in rheumatoid arthritis (RA), inflammatory bowel disease (IBD) and even psoriasis patients receiving TNF inhibitors.
A systematic review of published cases of TNF-α inhibitor-induced psoriasis identified 88 articles with 216 cases of new-onset TNF-α inhibitor-induced psoriasis.
The mean age at psoriasis onset was 38.5 years. The most commonly affected were Crohn disease (40.7%) and rheumatoid arthritis (37.0%). Onset of TNFi-induced psoriasis occurred after an average of 14.0 months of TNFi therapy, with 70% noting onset within the first year.
Affected patients were primarily taking infliximab (62.5%) or adalimumab (21.8%) and few were treated with etanercept (14.4%).
Skin presentations were mixed in 26.9% of patients The most common presentations included plaque (44.8%), palmoplantar pustular (36.3%) psoriasism and psoriasiform dermatitis (19.9%), severe scalp involvement (7.5%), and generalized pustular psoriasis (10.9%). The lesions were equally distributed on the soles, extremities, palms, scalp, and trunk. Histopathology revealed primarily plaque psoriasis (54.9%) and pustular psoriasis (33.3%).
Although topical therapy was used in most, the most effective regimens were: 1) 47.7% TNFi discontinuation lead to resolution or improveent in 94%; 2) 36.7% switched TNFi inhibitors and nearly 55% improved or resolved; and 3) 32.9% continued therapy with 33% resolved and 57% improving their psoriasis.
Mechanisms underlying this adverse event are not well understood.
The authors suggest that skin-directed therapies works n many cases does and that cessation of TNFi treatment will be needed in a minority of patients.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.