B Cell Targeting in Idiopathic Thrombocytopenic Purpura Save
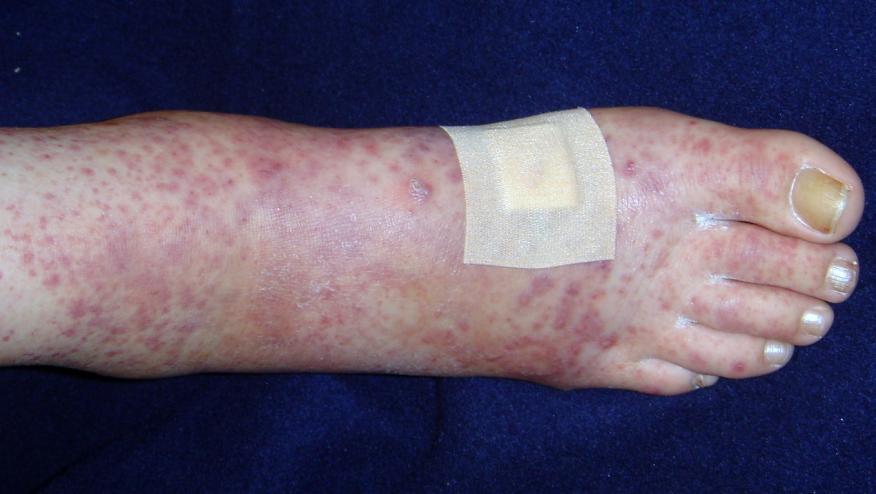
The New England Journal of Medicine has published a phase III clinical trial showing that a B cell targeting monoclonal antibody, ianalumab, was effective in more than half of the patients with primary immune thrombocytopenia (ITP). Moreover, treatment maintained safe platelet counts without serious bleeding episodes for at least one year.
ITP is an autoimmune condition where the body’s immune cells mistakenly attack platelets, the blood cells responsible for clotting. It affects about 50,000 people in the U.S. and can be diagnosed at any age. ITP is associated with abnormal bleeding from the skin and mucous membranes—including nosebleeds, gum bleeding, and/or heavy menstruation—that can be severe when platelet counts are particularly low. ITP also contributes to easy bruising and fatigue.
Some patients with ITP do not require treatment, but for those with low platelet counts or repeated or severe bleeding, initial treatment involves steroids, which work well for some patients. However, for patients who continue to have bleeding issues or low platelet counts with—or after tapering off—steroids, another form of treatment is required. While there are currently three FDA-approved second-line therapies for ITP, they all generally require treatment for life, either in the form of a daily pill or weekly injections, which come with their own side effects and costs.
The double-blind, multicenter clinical trial (called the VAYHIT2 study) randomized 152 adult patients with ITP to three arms: a higher-dose of ianalumab (50 patients), a lower-dose of ianalumab (51 patients), or a placebo (51 patients). Ianalumab works by targeting the B-cell-activating factor (BAFF) receptor, resulting in a depletion of autoreactive B cells that are responsible for the anti-platelet antibodies that cause ITP. Patients were eligible for the study if they had already experienced a relapse after steroids or if their ITP did not respond to treatment with steroids. Ianalumab was given intravenously once a month for four months, and because it takes time to start working, all patients also received eltrombopag, one of the pills currently approved for second-line treatment. Eltrombopag is normally taken indefinitely but was intended to be tapered off and stopped for this study.
The study measured “time to treatment failure,” defined as a low platelet count, the need for additional ITP therapy, inability to taper or discontinue eltrombopag, or death. The estimated probability of avoiding treatment failure at 12 months was 54.2 percent in the high-dose arm and 50.5 percent in the low-dose arm, versus only 30 percent of patients in the placebo arm. Additionally, when platelet counts were measured at six months (two months after the last dose of ianalumab), 62 percent of patients in the high-dose treatment arm had stable platelet counts, versus only 39.2 percent of patients in the placebo arm.
Additional clinical trials for ianalumab are ongoing, including in studies for other autoimmune conditions, and it is not yet FDA-approved for patients. The researchers will continue to follow the patients from this study to track long-term response to treatment.
The study was funded by Novartis.
New England Journal of Medicine 10.1056/NEJMoa2515168









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.