Baricitinib Efficacy in Alopecia Areata Save
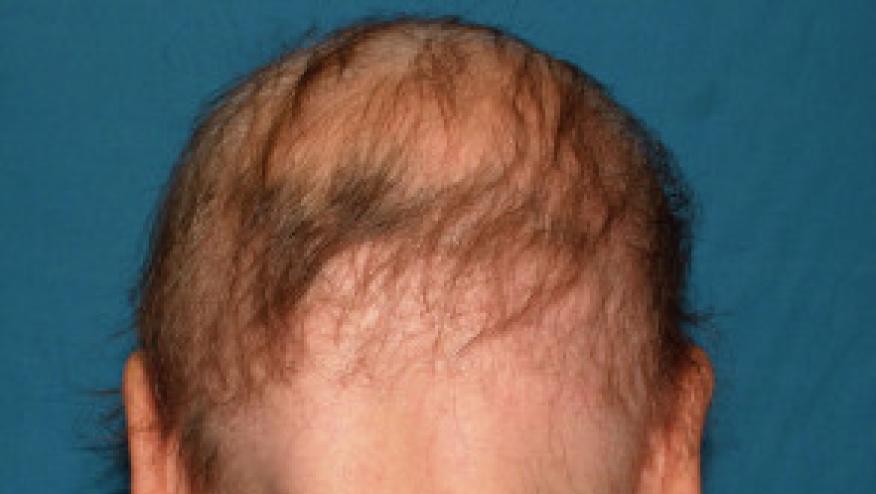
The NEJM has published the results of the BRAVE-AA1 and BRAVE-AA2 trials, demonstrating that baricitinib is effective at regrowing hair in alopecia areata (AA) patients, thus paving the way for future regulatory approval for this difficult to treat disorder.
Alopecia areata is an difficult to manage autoimmune condition and in the US has a lifetime prevalence of roughly 2%.
The two, phase 3, randomized, placebo-controlled trials treated adults with severe alopecia areata with either placebo or 2 mg or 4 mg baricitinib daily. The Severity of Alopecia Tool (SALT) score had to be > 50 (range, 0 [no scalp hair loss] to 100 [complete scalp hair loss]). The primary outcome was a SALT score of 20 or less at week 36.
A total of 1200 patients were enrolled in the BRAVE-AA1 and BRAVE-AA2 trials.
The week 36 endpoint results (SALT score < 20) in the BRAVE-AA1 and BRAVE-AA2 trials were (respectively) :
- 4-mg baricitinib - 38.8% and 35.9%
- 2-mg baricitinib - 22.8% and 19.4%
- Placebo - 6.2% and 3.3%, respectively. (P<0.001 for each dose vs. placebo). In
Adverse events included acne, elevated CK levels and increased levels of low- and high-density lipoprotein cholesterol were more common with baricitinib than with placebo.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.