Biomarkers Predictive of a Deucravacitinib Response in Psoriatic Arthritis Save

A phase II trial demonstrating the clinical efficacy of deucravacitinib, a TYK2 inhibitor, in psoriatic arthritis (PsA) suggests specific biologic effects identified by biomarkers may predict clinical responses.
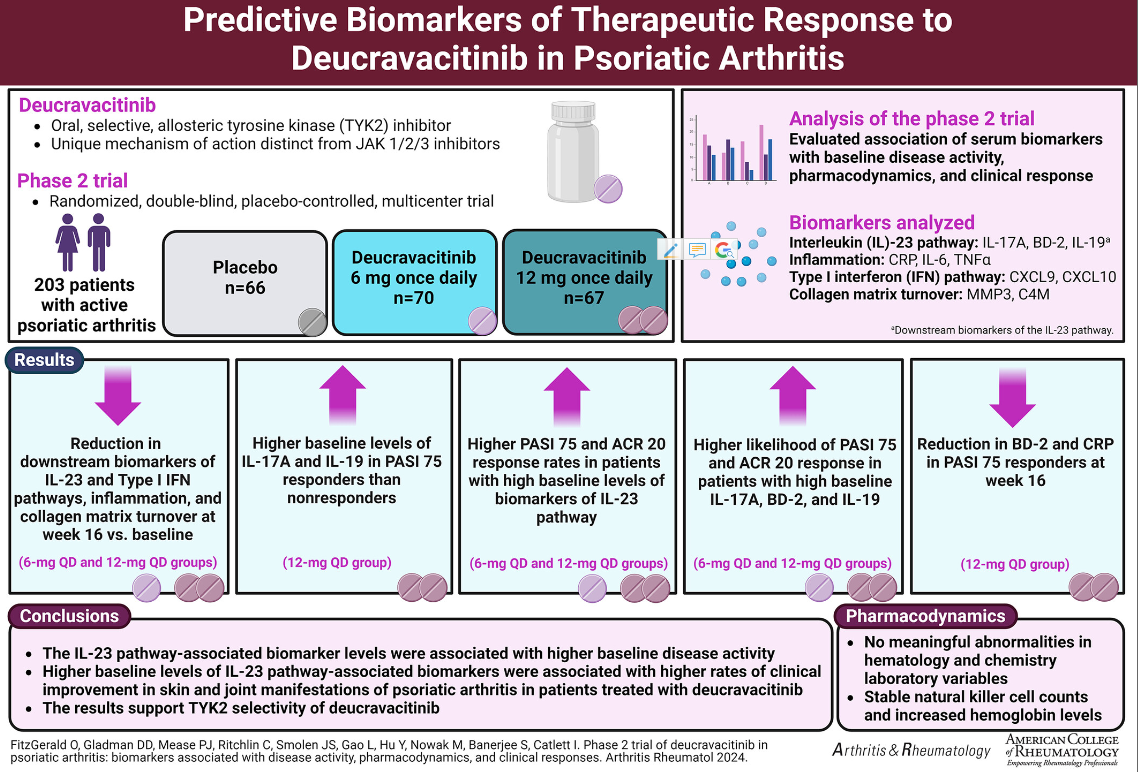
The phase 2 trial (NCT03881059) was previously published in 2022 and included 203 PsA patients randomized to to placebo, deucravacitinib 6 mg once daily (QD), or deucravacitinib 12 mg QD. At week 16, ACR-20 responses were significantly higher with deucravacitinib 6 mg (52.9%) and 12 mg once a day (62.7%) compared to placebo (31.8%). Both deucravacitinib doses were significant superior to placebo (p≤0.05).
Serum biomarkers were assess at several time points and included those of IL-23 pathway (IL-17A, BD-2, and IL-19), Type I interferon pathway, inflammation, and collagen matrix turnover.
At baseline IL-17A, BD-2, and IL-19 had a modest association with PASI scores (r=0.4, r=0.56, and r=0.5, respectively).
At week 16 (primary endpoint) IL-17A, BD-2, IL-19, CXCL-9, CXCL-10, CRP, MMP3, and C4M levels were significantly reduced in patients on In deucravacitinib (P<0.01).
Higher levels of IL-23 associated biomarkers predicted higher PASI 75 and ACR 20 response rates in deucravacitinib-treated patients. Better PASI 75 response rates were seen in deucravicitinib treated PsA patients with high baseline IL-17A (OR: 15.76) and BD-2 (OR: 15.41).
Deucravacitinib inhibition of TYK2 signaling affects IL-23 and Type I IFN pathway, as well as collagen matrix turnover. These biomarkers may predict treatment responses to deucravacitinib










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.