Combination biologics in PsA: are we there yet? Save
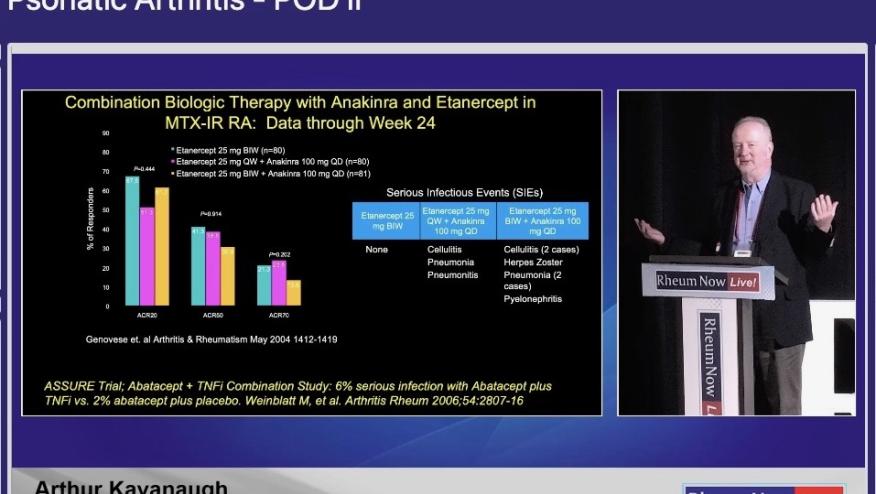
The benefits of combination therapy are well known in rheumatoid arthritis with the use of conventional and biologic DMARDs, yet data has been conflicting in psoriatic arthritis (PsA). Despite various and efficacious options for management of PsA there is still an unmet need for patients with inadequate responses to current therapies or difficult-to-treat disease. The role of dual biologic therapy targeting different inflammatory pathways has become an area of great interest in the field.
At the recent RheumNow Live 2025 meeting, the Psoriatic Arthritis pod presented important information and key takeaways.
While not a standard practice due to concerns for increased infection risks and limited long-term safety data, combination biologic therapies in PsA are being explored on emerging research. Promising data obtained from the results of VEGA study, a phase 2 trial which evaluated combination guselkumab (IL-23i) with golimumab (TNFi) in ulcerative colitis showed combination therapy was most effective than each individual therapy with comparable safety risks.
In the case of PsA, no clinical trials have been published evaluating the efficacy and safety of this treatment approach, however, there is evidence based on case reports and retrospective studies favoring the combination of: IL-12/23i+TNFi, IL-23i+TNFi, and IL-17i +TNFi without alarming adverse events reported.
Despite of lacking clinical trials, it seems that combination with apremilast (PDE4i), while not necessary a biologic, has been frequently utilized clinical practice. Additionally, the emergent therapies approved for management of psoriasis which include the tyrosine kinase 2 inhibitors (TYK2) have shown favorable responses in efficacy and safety making them an interesting combination with already approved biologics for PsA.
To date, many questions remain unanswered regarding this novel modality of therapy, including the choice of the most efficacious combination, the most appropriate timing, frequency, and duration of treatment. As well as the potential strategies to mitigate adverse events.
It seems reasonable to consider combination biologic therapies in management of PsA when associated with comorbidities such as inflammatory eye disease, inflammatory bowel disease and axial spondyloarthritis, or in the treatment of different inflammatory conditions such as asthma or atopic dermatitis.
Nevertheless, the application of such strategies in PsA requires further investigation through rigorous clinical trials to assess efficacy and safety.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.