DMARD Responses in Localized Scleroderma Save
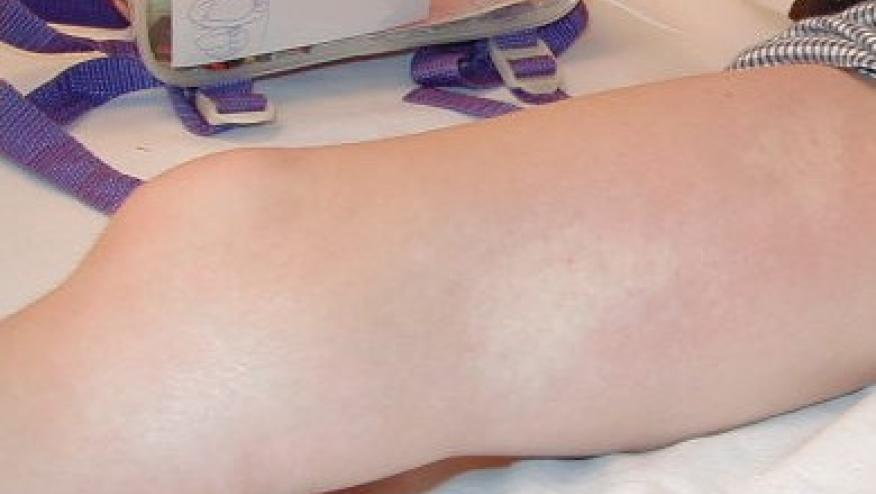
JAMA Dermatology has published the results of a juvenile localized scleroderma (JLS) trial showing that mycophenolate mofetil (MMF) and methotrexate (MTX) are equally effective in treating JLS, noting low flare rates and possibly better tolerability with MMF.
There are no FDA approved treatments for juvenile localized scleroderma (JLS), a rare disease, even though MTX has become first-line therapy. This trial sought to test if the antecdotal reports of MMF efficacy in JLS are true by comparing outcomes with MTX.
This was a single center, retrospective cohort study of JLS (enrolled in the National Registry of Childhood Onset Scleroderma) patients under age 18 years and a diagnosis of JLS. Enrolled patients could have received either MTX monotherapy, MMF monotherapy, or combination therapy (CT). JLS disease activity and Flares were measured using the Localized Scleroderma Cutaneous Assessment Tool.
From a total of 114 JLS patients (67%, median onset 8.3 years), patients were treated with either MTX (60%), MMF (25%), or combo (CT) (16%). Groups were well matched, althought the MMF group had a longer disease duration.
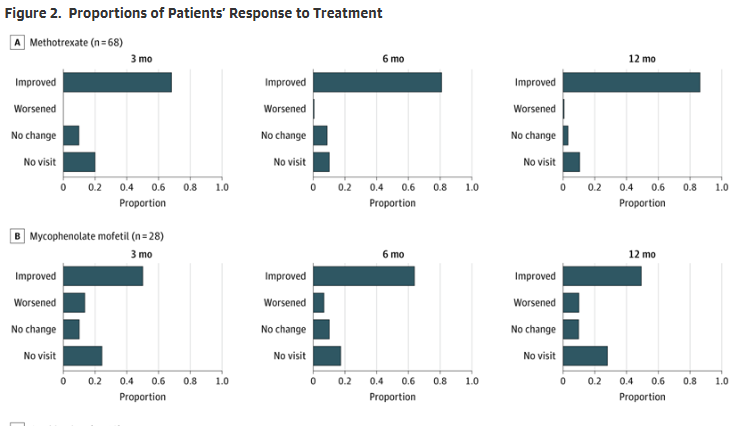
A Kaplan-Meier analysis showed no significant difference in disease flare rate (HR 0.85; 95% CI, 0.51-1.33). MTX patients did have more fatigue (47% vs. 11%, P = .001) and nausea (60% vs. 7%; P = .001) compared to the MMF group.
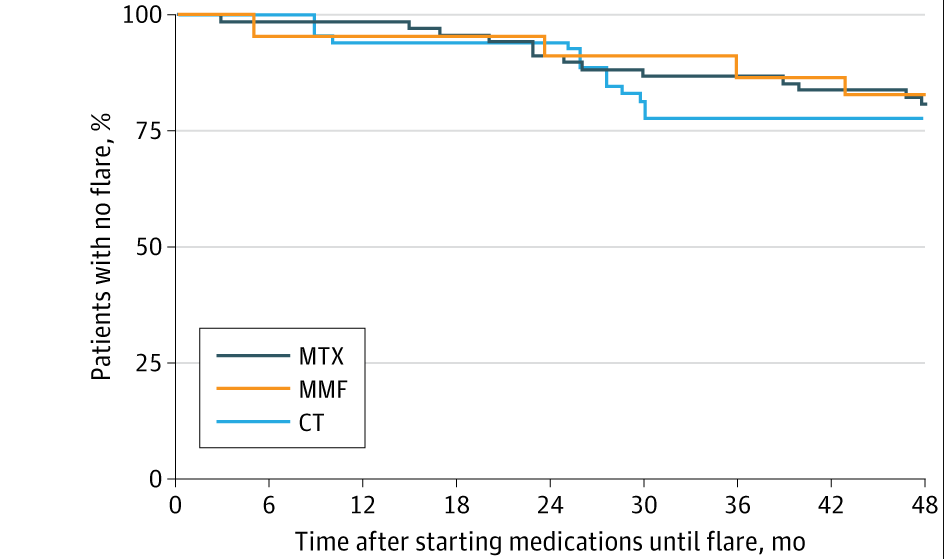
MMF appears to have equivalent efficacy compared to MTX in reducing disease activity, with comparable flare rates and improved tolerability. These observational findings support the need for a prospective, randomized, noninferiority trials are warranted to confirm these results and guide future treatment recommendations.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.