FDA Invites Patient Input on Systemic Sclerosis Drug Development Save
On October 13th, 2020, FDA held a virtual public meeting on "Patient-Focused Drug Development for Systemic Sclerosis". While there have been several promising new agents for systemic sclerosis, many have failed - with all noting the difficulties in appropriate outcomes and endpoints for clinical trials. With this in mind, it seems appropriate that the FDA was interested in hearing patients’ perspectives on systemic sclerosis and new drug development.
Theresa Mullin, PhD began the meeting with an overview of FDA’s "Patient-Focused Drug Development Initiative", a program designed to capture and incorporate patients’ experiences, perspectives, needs, and priorities in the course of drug development and evaluation. With patients as experts of the condition that they live with, they are uniquely positioned to inform those working in drug development and evaluation.
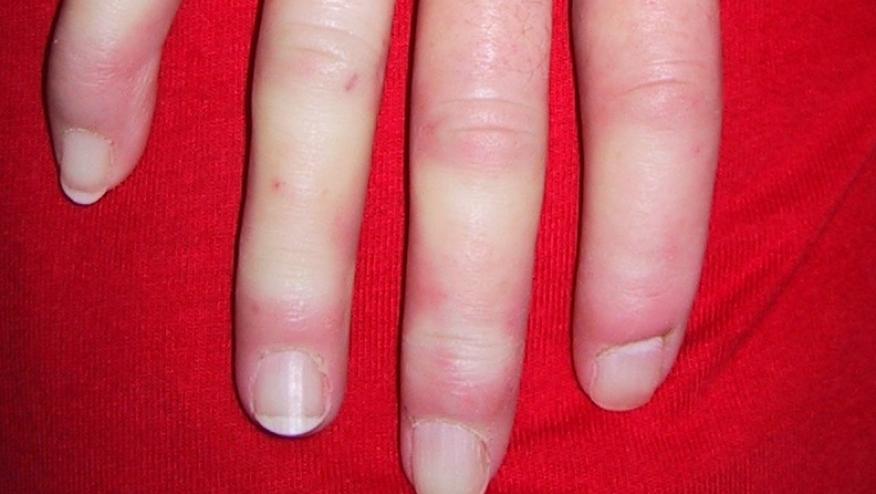
Dr. Dinesh Khanna, provided a clinical and scientific background on Systemic Sclerosis and it's therapies. There are nearly 200,000 scleroderma patients in USA with 75,000-80,000 of these having systemic sclerosis. He estimated that there are about 6,000 new systemic sclerosis cases each year. Dr. Khanna said, "The main take away points from the PFFD SSc meeting was a clear consensus among the panelists about an urgent need for effective drugs in systemic sclerosis and lack of incorporation of outcome measures that affect people with systemic sclerosis".
He noted that "the patients highlighted [different clinical outcomes, such as] pain, calcinosis, gastrointestinal involvement, and Raynaud’s phenomenon as main symptoms, yet the ongoing trials tend use skin score and forced vital capacity as approvable end points".
While panel representation was diverse, he noted "they did not have representation of early diffuse systemic sclerosis (where involvement of skin can be associated with pruritis, tenderness, and allodynia of the skin), and there was a lack of men on the panel".
Two main Topic Areas and questions were posed to the discussants
Topic 1: Health effects and daily impacts that matter most to patients; Questions to patients
- Which aspects of systemic sclerosis have the most significant impact on your life?
- Are there specific activities that are important to you but that you cannot do at all or as fully as you would like because of your systemic sclerosis?
- How has your systemic sclerosis changed over time?
- What worries you most about your systemic sclerosis?
- If you could change one thing about your systemic sclerosis, what would it be?
Topic 2: Patients’ perspectives on current approaches to treatment
- What are you currently doing to help treat your systemic sclerosis?
- How has your treatment regimen changed over time, and why?
- What symptom would you most like to be improved or resolved by treatment?
- How well does your current treatment regimen treat the most significant aspects of your systemic sclerosis?
- What are the most significant downsides to your current treatments, and how do they affect your daily life?
- Aside from a complete cure for your systemic sclerosis, what specific things would you look for in an ideal treatment for your systemic sclerosis?
- What factors do you consider when making decisions about selecting a course of treatment?
- Efficacy and safety are important for any treatment. When you think about a treatment for your disease:
- Does it make a difference whether: i. the product might improve your most bothersome symptoms or ii. whether the product might preserve organ function, or iii. would you consider improvement in either of those areas equally worth the same level of risk?
- Does your acceptance of potential safety risks go up with the potential effectiveness of a product?
- What degree of risk are you willing to accept for an effective treatment? Risks associated may range from mild to severe and lifelong (such as life-long immunosuppression, risk of potential cancer associated with treatment).
- If you had to choose between two products, which would you prefer? A very safe product that works somewhat, or a less safe product that works really well?
Closing comments included, patient concerns about:
- pain from Raynauds, digital ulcers and calcinosis
- fatigue can be severe and debilitating
The FDA will accept patient comments until Dec. 15, 2020, at which time comments will be compiled into a summary report.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.