SGLT2 inhibitors: The new hype in SLE and LN management? Save
Inhibitors of SGLT2 have been shown to reduce MACE (nonfatal myocardial infarction and nonfatal stroke, cardiovascular death) in patients with type 2 DM and established cardiovascular disease. In patients with chronic kidney disease at risk of progression, SGLT2 inhibitors have shown reduction in risk of eGFR decline and hospitalization. In SLE, clinical trials have yet to explore the possible role of SGLT2i in improving renal outcomes among those with lupus nephritis. A couple of abstracts presented during the meeting tackled the topic of SGLT2 inhibition in SLE.
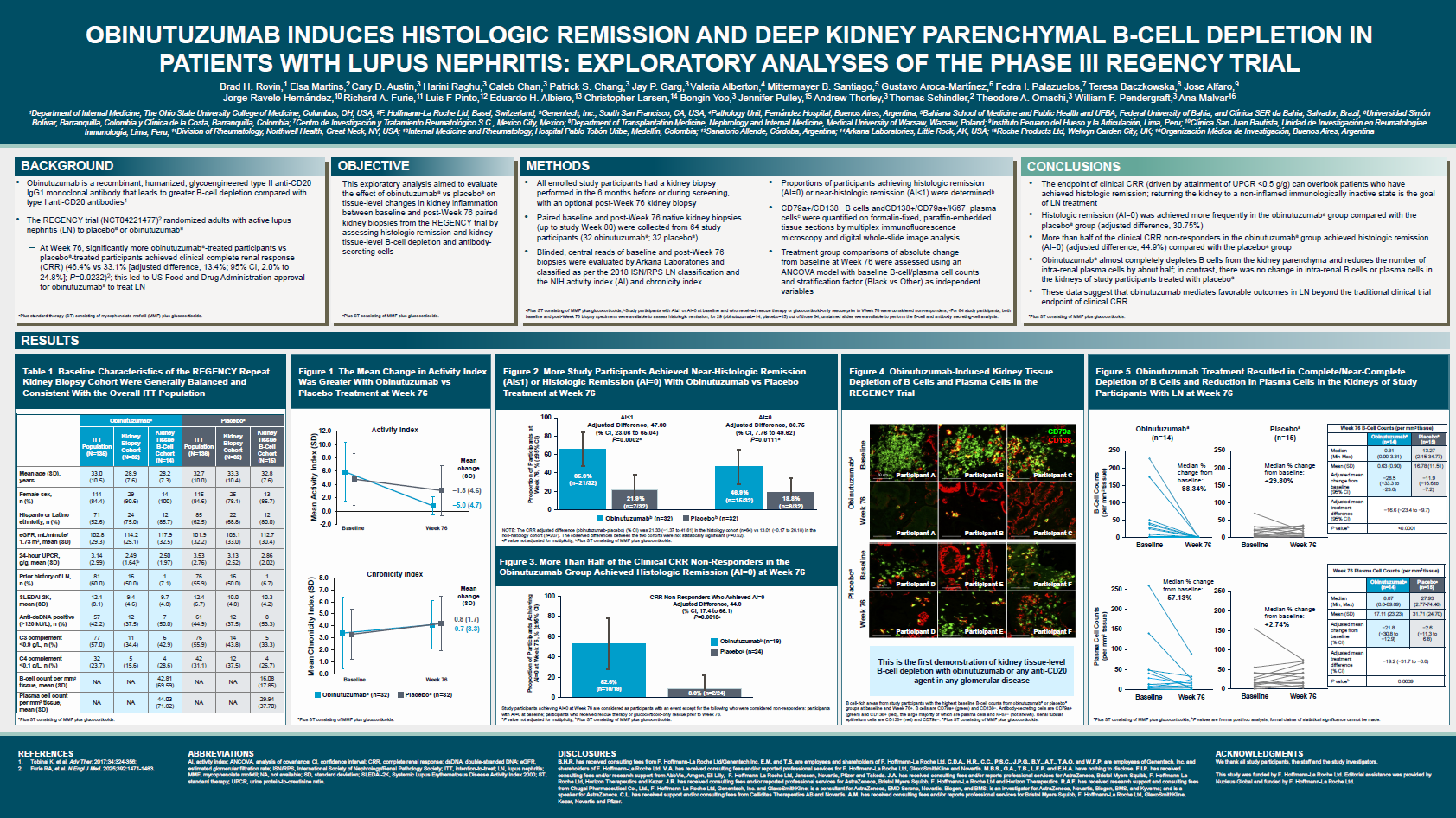
In abstract 1492, Professor Michelle Petri and her colleagues presented data on their early experience with SGLT2i in lupus patients. Forty-five patients with SLE who were started on SGLT2i were included, with the drug being started for standard of care (DM, CKD or both). Prior to starting SGLT2i, the mean follow-up time was 1.9 years while the mean follow-up time after starting SGLT2i was 1.1 years. The primary outcomes included changes in eGFR and the urine protein-creatinine ratio. Results show that prior to starting SGLT2i, the average annual change in eGFR was a decline of 3.1 (p=0.0014) and after starting SGLT2i, the average annual change in eGFR was a decline of 0.9. Observable differences were noted between the two groups but these were not statistically significant (p=0.27). Meanwhile, the average annual change in UPCR was an estimated increase of 0.40 prior to starting SGLT2i and after starting SGLT2i, the average annual change in UPCR was estimated to be a decline of 0.37. Again, the differences were not statistically significant. These results suggest a marginal benefit of SGLT2 inhibition in SLE patients but given a small sample size, more data and a longer follow-up period are needed to elucidate a definitive conclusion.
Abstract 1579 by Dr. April Jorge and colleagues reported the impact of SGLTi vs. DPP4i on renal and CV outcomes among patients with SLE and LN using a target trial emulation network from TriNetX, a large US multi-center HER-based SLE cohort. Trial outcomes included renal progression; defined as a decrease in eGFR by >30% or new-onset ESRD and major adverse cardiac events (MACE); defined as CV death or hospitalization for ischemic stroke, myocardial infarction, heart failure, or venous thrombosis; and genital infections. Results show that SGLT2i was associated with lower risk of renal progression (HR 0.71 [95% CI 0.51-0.98]) and lower risk of MACE (HR 0.69 [95% CI 0.48-0.99]) compared with DPP4i use. In the subgroup with LN, the risk of MACE was also lower with SGLT2i (HR 0.58 [95% CI 0.34-0.99]). Consequently, the use of SGLT2i inhibitors was associated with higher genital infections.
Another abstract (0590) by Dr. Jui-En Lo and colleagues compared the safety and efficacy of SGLT2i vs. DPP4i in SLE patients with comorbid type 2 DM using an emulated clinical trial. After a follow-up period of over 7.95 (±14.5) mean years, patients on SGLT2i had significantly reduced risks of developing AKI (HR 0.65; 0.56-0.75), CKD (HR 0.74; 0.66-0.82), ESRD (HR 0.40; 0.27-0.59), and heart failure (HR 0.80; 0.71-0.90) compared to those patients who received DPP4i. Patients on SGLT2i also had decreased future emergency visits (HR 0.82; 0.77-0.88) and hospitalization (HR 0.68; 0.53-0.89). Although SGLT2i use was associated with increased risk of genital infections (HR 1.29, 1.12-1.49), risks for UTI and severe sepsis were low [(HR 0.90; 0.75-0.91; HR 0.61; 0.45-0.84)], respectively.
Findings of these studies look promising but additional investigations may be required to validate the conclusions. Many more questions about the role of SGLT2i in SLE may arise more than answers at this point and I’m pretty sure that we’re just starting to experience the SGLT2i hype.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.