2025 ACR Guidance on Diagnosis and Management of VEXAS Save

The ACR has published a formal international consensus guidance on VEXAS as a resource for clinicians seeking to understand the disease and its management.
VEXAS is a rare genetic disorder; the acronym stands for Vacuoles E1 enzyme X-linked autoinflammatory somatic syndrome (VEXAS) and is associated with somatic mutations in the UBA1 gene. VEXAS presents with a combination of inflammatory and hematologic manifestations, leading to increased morbidity and mortality.
An international multidisciplinary panel of VEXAS experts was established to educate and provide guidance to health care providers. This guidance covers several several key topics: (1) clinical features of VEXAS, (2) UBA1 screening methods, (3) the diagnosis of myelodysplastic syndromes (MDSs) in patients with VEXAS, and (4) prognosis and management.
First described in 2020, there are more than 450 cases documented, with a variety heterogeneous manifestations. The prevalence of VEXAS is estimated to be nearly one million individuals worldwide; the UBA1 pathogenic variants are seen in 1 in 13,591 individuals.
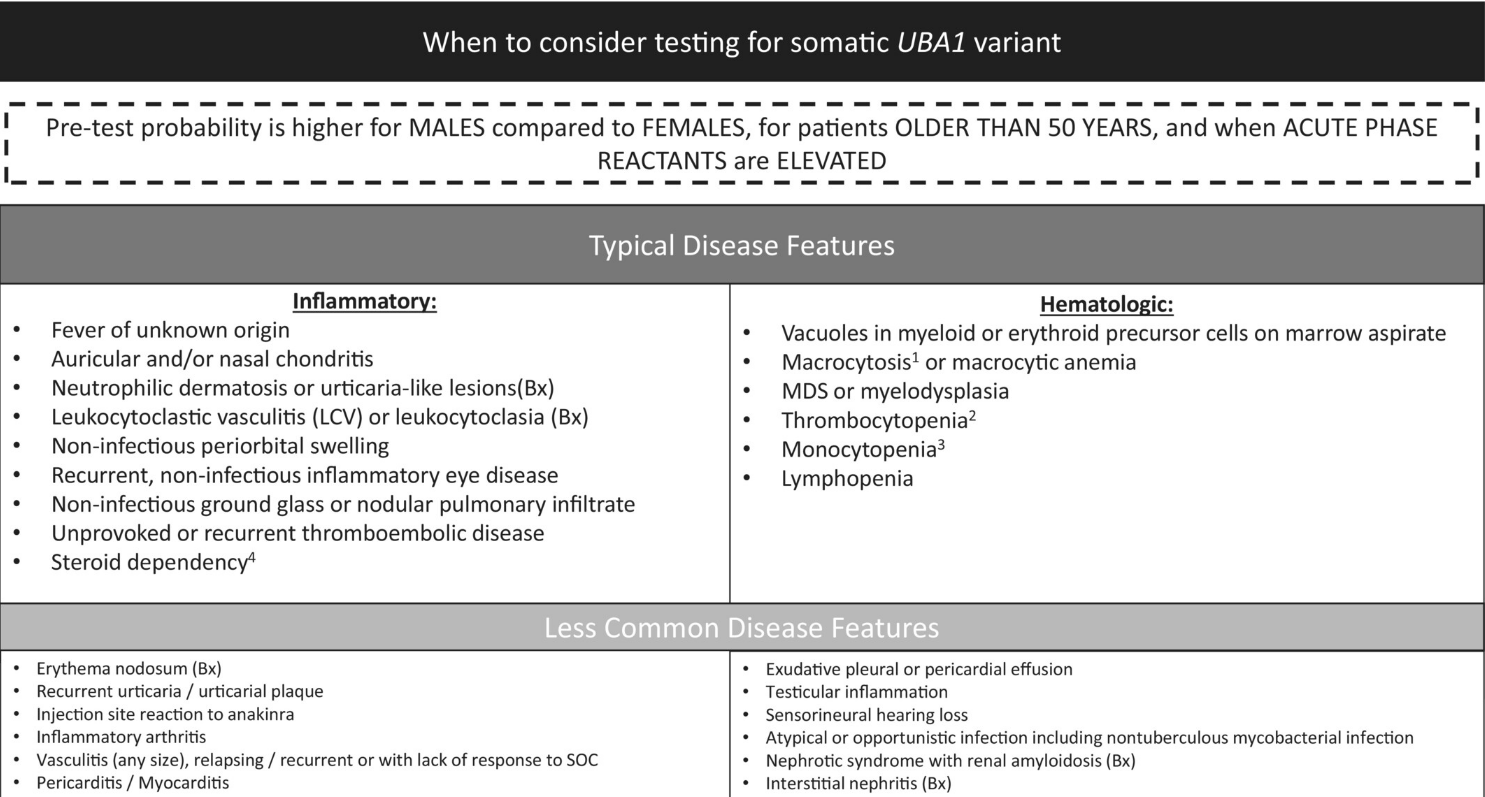
VEXAS can be a significant diagnostic challenge, as its features often mimic other established rheumatologic and hematologic diseases. Genetic testing and identification of patients with VEXAS is crucial diagnosis and to guide appropriate management.
This guidance was established by literature review and consensus voting by a worldwide panel of experts.
The following statements are the products of 4 work groups
Group 1: clinical and laboratory features
- VEXAS is a hematoinflammatory disease characterized by a spectrum of rheumatologic, dermatologic, and hematologic features
- Persistently elevated markers of inflammation with a combination of skin, ocular, lung, or cartilage manifestations and/or cytopenia are suggestive of VEXAS
- Macrocytic anemia is a common hematologic abnormality in VEXAS but may not always be present
- Vacuoles present in early erythroid or myeloid precursors from bone marrow aspirates are suggestive of, but not diagnostic for, VEXAS
- VEXAS is not a heritable disease
- Most cases of VEXAS have been reported in men older than 50 years
Group 2: UBA1 mutation screening
- The majority of patients with typical VEXAS have missense or splice site mutations at exon 3 of UBA1
- A variety of sequencing methods can be used to diagnose VEXAS depending on resources and expertise
- NGS has increased sensitivity to detect low-level somatic variants with coverage of all coding or splice site regions
- Targeted testing for exon 3 variants can be used as an alternative to NGS with the understanding that such modalities may miss somatic variants with low VAF (Sanger sequencing) or variants outside of exon 3 (Sanger sequencing and ddPCR)
- Active treatment may affect the accuracy of genetic testing by decreasing mutation level
- Somatic mutations defining VEXAS are detected in peripheral blood or bone marrow–derived DNA
- If clinical suspicion is high and genetic testing for mutations in UBA1 at exon 3 is negative on the peripheral blood, clinicians should ensure that testing covers the complete gene and consider testing a bone marrow sample
Group 3: diagnosis of MDS in patients with VEXAS
- Bone marrow examination is recommended in patients with VEXAS with associated cytopenia to exclude an associated hematologic neoplasm
- Karyotype and NGS for coexisting somatic mutations should be performed with bone marrow examination
- Interpretation of bone marrow is challenging in VEXAS due to the frequent presence of signs of dysplasia, without meeting existing criteria for myelodysplastic syndrome diagnosis based on the WHO and ICC 2022 classifications
- The somatic clonal landscape in VEXAS is dominated by mutations in DNMT3A and TET2, generally without other driver mutations
Group 4: outcomes, prognostic factors, and management
- Patients with VEXAS should be managed by a multidisciplinary team with consideration of referral to an expert center when available evidence
- Disease activity is defined by inflammation or worsening bone marrow failure
- The goals of treatment include controlling inflammation; preventing and treating bone marrow failure; preventing and treating secondary medical complications arising from implemented treatments; and improving quality of life
- Infections, thromboembolic embolic disease, and treatment-related comorbidities are common in VEXAS and can mimic disease activity
- Flare of disease can be defined as recurrence of clinical or laboratory signs or symptoms of VEXAS
- Glucocorticoids are usually needed to control inflammation in patients with VEXAS and should be tapered slowly to achieve the minimal dose required to control disease activity
- Medications that target inflammatory pathways (eg, JAK inhibitors, IL-6 inhibitors) are more effective than conventional DMARDs (eg, methotrexate, azathioprine) or B cell–directed therapies (eg, rituximab)
- Medications that reduce or eliminate the clonal burden of disease (eg, azacytidine) can be an effective treatment in some patients with VEXAS syndrome
- Allogenic hematopoietic stem cell transplantation may be a curative treatment but should be reserved for select patients with VEXAS after a careful hematologic evaluation
- Prophylaxis against opportunistic infections, prevention of thromboembolic disease, and minimization of side effects related to chronic glucocorticoid therapy should be considered in all patients with VEXAS
Future clinical trials will be needed to identify the best treatment for patients with VEXAS
Join The Discussion
Great scientific work










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.