STOP-RA: Hydrochloroquine Fails in ACPA+ Arthralgia Save
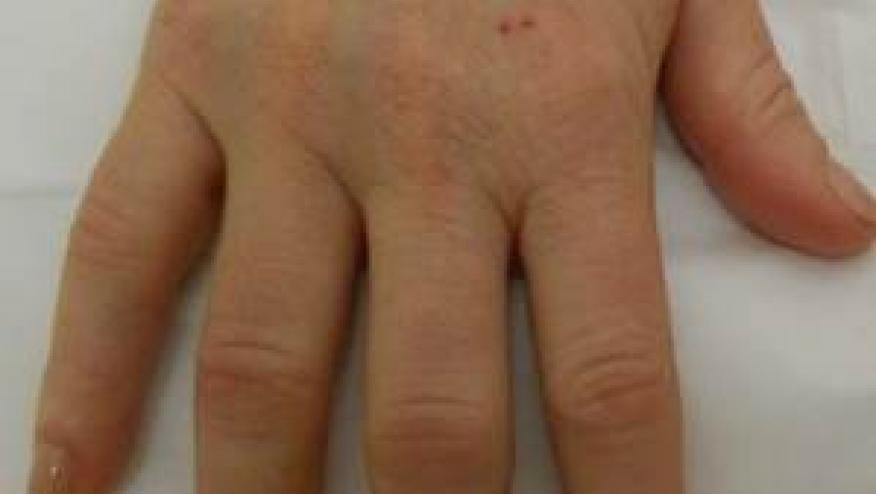
Deane et al has published the results of the STOP-RA trial, demonstrating that 12 months of hydroxychloroquine (HCQ) did not prevent the development of clinical RA at 36 months.
The fate of Individuals elevated anti-cyclic citrullinated peptide (anti-CCP) antibodies and polyarthralgia is uncertain and it is unknown if safe first line therapies may thwart the subsequent development of rheumatoid arthritis (RA). Moreover, there are no pharmacologic interventions have been approved for the prevention of RA or ‘at-risk’ individuals.
A phase 2 randomized trial, sought to evaluate the potential efficacy of hydroxychloroquine (HCQ) in at-risk for RA individuals, thus those with anti-CCP ≥2 times the upper limit of normal (ULN) and arthralgia were randomized to receive HCQ or placebo for 12 months, with up to 24 months of post-drug follow-up. The primary outcome was the development of clinical RA, at 36 months. This trial was conducted during the COVID-19 pandemic and enrollment was slow.
A total of 144 were randomized (71 HCQ and 73 placebo). At 12 months, clinical RA occurred in 21 of 69 (30.4%) participants in the HCQ group and 24 of 73 (32.9%) in the placebo group. At 36 months, the risk of clinical RA at 36 months was 0.336 with HCQ and 0.394 with placebo was not significantly different (difference -0.058; 95% confidence interval -0.336 to 0.220; P=0.52).
The occurrence IA, and severity of joint symptoms, and adverse events did not differ between groups.
(Funded by the National Institute of Allergy and Infectious Diseases; ClinicalTrials.gov number, NCT02603146.)








If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.