Methotrexate in PsA Save
Until the publication of the SEAM trial1, evidence in the medical literature for the efficacy of the most commonly used drug for psoriatic arthritis (PsA) worldwide, methotrexate (MTX), has been lukewarm at best. Yet we all employ it commonly, either as monotherapy or in combination with biologic or targeted synthetic DMARD treatment. It is inexpensive and widely available, and only modestly toxic.
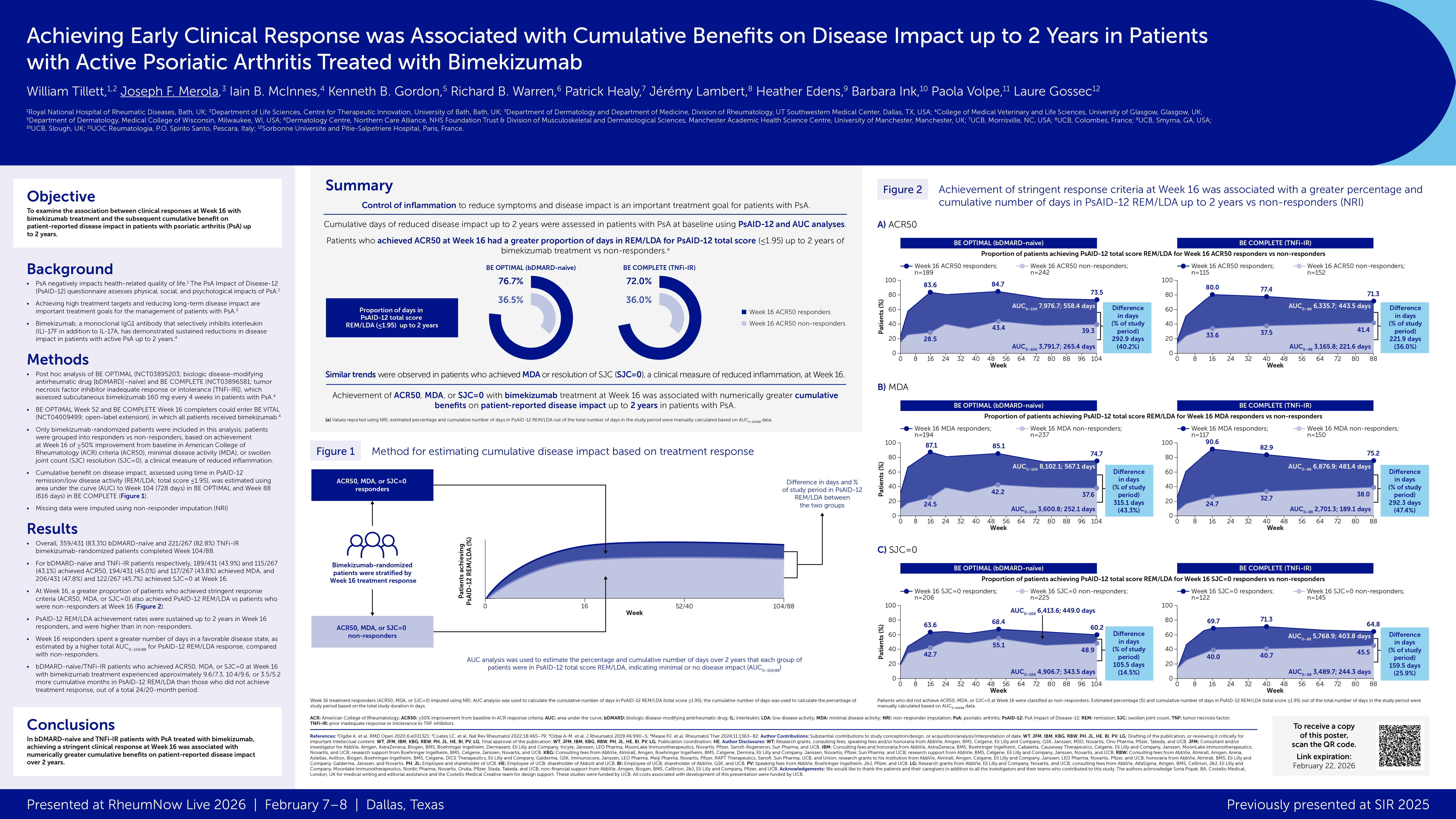
In 1984, Willkens published a small trial of 37 patients treated with MTX 7.5 or 15 mg per week vs. placebo2. The only improvements in the MTX arm were physician global and psoriasis skin lesion score – thus a “failed” trial.
In 2012, Kingsley published a larger placebo-controlled trial conducted in the United Kingdom2. The key endpoints of ACR 20, DAS28, Psoriatic Arthritis Response Criteria (PsARC), and Psoriasis Area and Severity Index (PASI)75, as well as tender and swollen joint count, did not show statistical separation of MTX and placebo, a few parameters did, such as patient and physician global assessment. It was acknowledged that the trial had problems: excessive number of dropouts from each arm, difficulty with recruitment and mainly patients with mild disease being studied. This, too, was considered a “failed” study.
Furthermore, as trials of biologics, such as TNF inhibitors were conducted with patients either on or off background MTX, the background use of MTX made no difference in clinical or radiologic outcomes, reinforcing the idea that there was little utility in using MTX. Yet for clinicians over the world, who had seen symptomatic improvement in at least a portion of their PsA patients. These results seemed counter to their experience and thus their practice continued, at least for the group of patients who were able to tolerate the drug without excessive nausea, fatigue, mouth sores, or hepatic-hematologic abnormalities and appeared to get some symptomatic benefit and who did not show evidence of progressive structural joint damage.
However, for patients who were able to access newer biologics and tsDMARDs, the majority of patients would move on to these more effective drugs, either as monotherapy, reassured that MTX background did not matter, or with MTX background if full benefit was not achieved with monotherapy application of the newer agent. Further, some clinicians will use MTX in a lower than “therapeutic” dose, e.g. 10 mg/wk, in order to take advantage of its potential role in reducing anti-drug antibody formation against therapeutically administered biologic medications.
The SEAM trial was the first “head-to-head” trial in PsA, including MTX-naïve, early PsA patients (median disease duration 6 months) randomized to MTX alone, etanercept (ETN) alone, or the combination of ETN and MTX. Not surprisingly, those treated in the two ETN arms did better is essentially every measure of arthritis, enthesitis, dactylitis, skin disease, function, quality of life, radiographic outcomes, and achievement of minimal disease activity (MDA) state, but MTX performed better than anticipated in all of these domains.
For example, whereas ACR 20 response was 65% and 60.9% in the ETN+MTX and ETN mono arms respectively, it was 50.7% in the MTX mono arm. MDA achievement was observed in 35.7% and 35.9% in the ETN+MTX and ETN mono arms respectively, and 22.9% in the MTX mono arm. Although to have full confidence about MTX performance it would have been better if a placebo group had been evaluated for comparison, nonetheless, to many clinicians, these results seemed consistent with our experience with MTX in practice.
A further observation was that unlike in earlier experience with similar trial designs in rheumatoid arthritis (RA), wherein the combination of MTX plus the biologic yielded better results than biologic monotherapy, the SEAM trial showed no difference between the ETN monotherapy and ETN+MTX arms of the study, thus appearing to exonerate clinicians from obligatorily recommending that MTX be used in combination with a biologic for best results.
The SEAM trial was published after the ACR-NPF PsA treatment guidelines were developed and published, but did support these recommendations3. Specifically, the guidelines recommended use of a TNF inhibitor prior to use of MTX, based both on efficacy as well as safety, but that MTX could be used as a second line therapy.
More recently, a trial known as the CONTROL study4 asked the following question: in PsA patients with inadequate response to MTX 15 mg/wk, does it make more sense to increase the dose of MTX to 25 mg or to instead combine adalimumab with MTX. In Part 1 of the study, not surprisingly, it became clear that many more patients achieved MDA at 16 weeks if adalimumab was added instead of increasing MTX dose. In Part 2 of the study, at 32 weeks, many of those not achieving MDA with adalimumab + MTX, were able to do so if adalimumab was intensified to weekly dosing, thus demonstrating value for this approach. And in those not achieving MDA on higher dose MTX, addition of adalimumab resulted in many patients achieving MDA.
In summary, MTX is a legitimate drug to use in PsA, either as a first systemic immunomodulatory, further down the line, or in combination with biologics and tsDMARDs. It is inexpensive, widely available, and very accessible. However, only a portion of patients will adequately respond and therefore will need to be on more effective medications, and many will not tolerate it because of side effects of nausea, fatigue, or hepatic-hematologic abnormalities. Thus, unlike in RA, wherein MTX is an important and commonly used combination medication, it appears to be a more optional medicine in PsA.
4. Mease PJ, Conaghan P, Tillett W, et al. Maintenance or Achievement of Minimal Disease Activity Following Therapy Optimization with Adalimumab or Methotrexate in Patients with Psoriatic Arthritis: Results from Part 2 of a Randomized, Open-Label Phase 4 Study. Arthritis and Rheum. 2020;72(S10):1035.
Join The Discussion
No declaration of conflicts: these must not count then '. PM has received grant support from AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Lilly, Novartis, Pfizer, Sun, and UCB; worked as a paid consultant for AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Sun, and UCB; and been paid as a speaker for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Lilly, Novartis, Pfizer, and UCB'. No risk of commercial bias then.
What this trial demonstrates is that suboptimal dosing of MTX (20mg po qwk) is not as effective as optimal dosing of ETN (50mg sc qwk).
Perhaps a trial of optimal dosing MTX 25mg sc qwk (starting dose, no titration) would yield a different result.
C Thorne Newmarket ON










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.