Zasocitinib (Tyk2 Inhibitor) Efficacy in Psoriatic Arthritis Save
A highly selective, tyrosine kinase 2 inhibitor zasocitinib (TAK-279), was studied in a phase 2b trial and found to be effective and safe patients with active psoriatic arthritis (PsA).
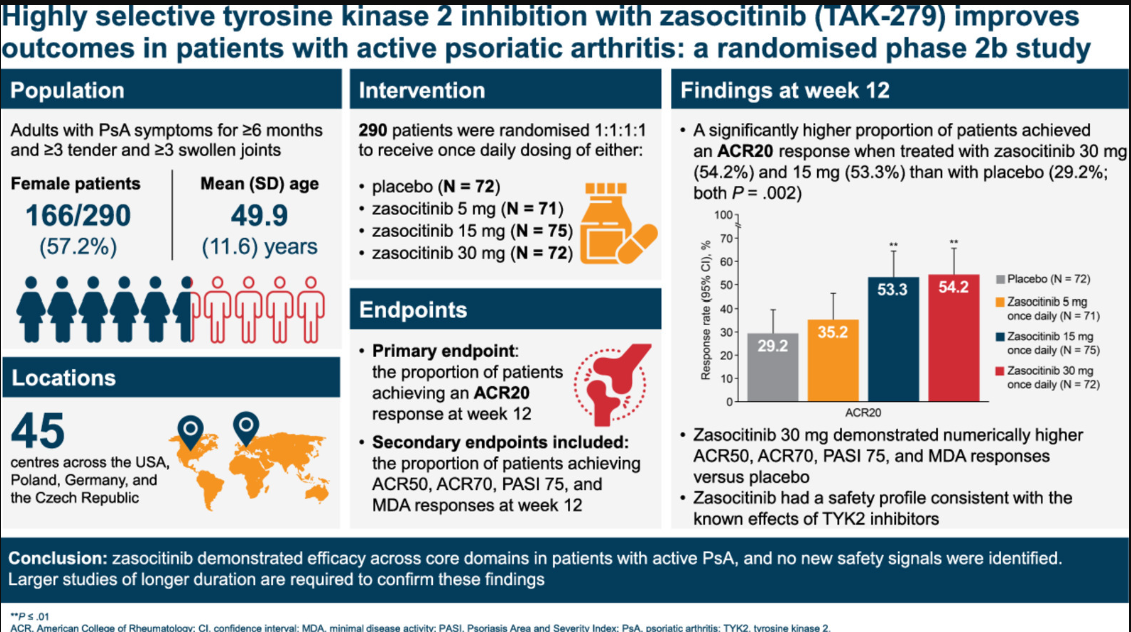
A phase 2b, randomised, multicentre trial used a double-blind, randomized, placebo-controlled, multiple-dose study design. They enrolled 2990 active PsA adult patients and randomized them to receive placebo, 30 mg, 15 mg, or 5 mg zasocitinib once daily for 12 weeks, with a 4-week safety follow-up. The primary endpoint was the ACR)20 response at week 12.
The week 12 ACR20 responses significantly favorted Zasocitinib:
- 30mg 54.2%;
- 15 mg 53.3%
- Placebo 29.2% (P = .002)
ACR50 responses were similarly significant (30 mg-26.4%; 15 mg-26.7%; placebo-9.7%). 30 mg zasocitinib had a higher number of ACR70 responses (13.9% versus 5.6%). Other superior secondary outcomes included the PASI 75 responses (45.7% versus 15.4%), and MDA (29.2% versus 12.5%).
No new safety signals or clear dose-dependent laboratory parameter changes were identified.
Zasocitinib 30 mg and 15 mg appears to be safet and effectiving in PsA, with no new safety concerns.
ADD THE FIRST COMMENT
Disclosures
The author has no conflicts of interest to disclose related to this subject










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.