Apremilast Continues to Look Good in Behcet's Disease Save
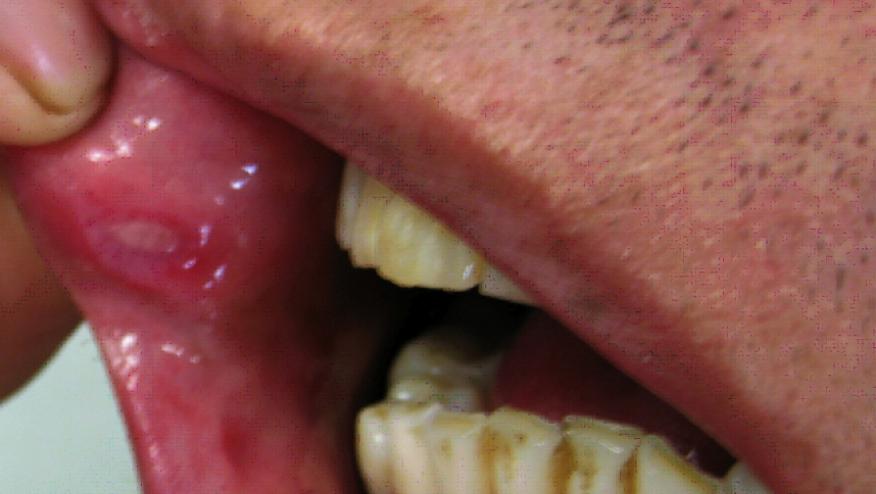
The 2018 American Academy of Dermatology (AAD) Annual Meeting features recent findings of a phase III trial of apremilast in active Behçet’s Disease, showing significant reductions in oral ulcers at week 12 for those treated with apremilast 30 mg twice daily.
The RELIEF trial studied 207 patients who were randomized to apremilast 30 mg BID or placebo. The trial’s primary endpoint was the area under the curve (AUC) for the number of oral ulcers at week 12.
The AUC was significantly for apremilast versus placebo (129.5 vs. 222.1; P0.0001). Significant improvements were also seen with apremilast in multiple secondary endpoints, including oral ulcer pain (P0.0001), overall disease activity (Behçet’s Syndrome Activity Score: P0.0001; Behçet’s Disease Current Activity Index: P=0.0335) and quality of life (P=0.0003).
Diarrhea was the most frequent adverse event (41.3 percent with apremilast, 19.4 percent for placebo). Otherwise the safety profile was consistent with the known safety profile of apremilast.
Celgene intends to submit its supplemental New Drug Applications for apremilast in active Behçet’s Disease with oral ulcers in the U.S. in the second half of this year.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.