No End in Sight for the Shingrix Vaccine Shortage Save
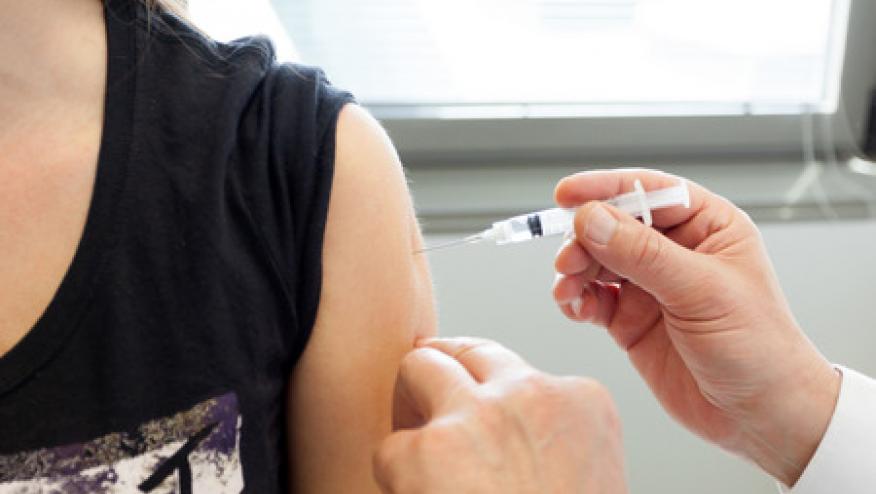
There is a national shortage of a new shingles vaccine, Shingrix, which is a problem for those who want to start the vaccine and those seeking to receive their second and final injection.
Since its FDA approval nearly a year ago, sales of the new vaccine have outperformed projections and are expected to approach $1 billion in revenue for 2018.
However, the growth and use of this two-dose Shingrix vaccine has been hampered by shortage and production delays.
There will be nearly 1 million cases of shingles each year in the United States; risk increases with age. GSK estimates that 115 million people in the United States ages 50 and older are eligible for the vaccine. GSK has not stated what their production capacity is to meet what appears to be a growing need.
Most insurance plans will cover Shingrix, though the copayment may vary. The cost per shot at CVS is $179, and the CDC list puts the price of each Shingrix dose in the private sector at $140 per shot.
Shingrix is a two-shot vaccine with the second injection given 2-6 mos. after the first. However, many are unable to find their second dose. Pharmacies are supposed to give priority to those patients. GSK says that nearly 70% of those starting the vaccine have received both injections.
US pharmacies are turning away those with a Shingrix prescription and most pharmacies are not keeping a waiting list since they have not been informed as to when this shortage will end.
GSK has announced they have been amping up their production, such that in December, they will move to a twice-monthly shipping schedule. However, no specifics on production were provided, other than 300,000 doses were shipped in November, and another 160,000 doses were shipped Dec. 3, 2018.
Shingrix is made in Belgium, and its production is already at maximum capacity. GSK says it takes 6-9 months to produce the vaccine.
The CDC says patients who wait longer than six months don’t have to start over. But they should get the second dose as soon as possible because the maximum immunity — more than 90 percent — is based on two doses. Protection stays above 85 percent for at least the first four years after vaccination, the CDC says.
GSK did not study how much immunity is provided by one dose.
The FDA website says this shortage began in May 2018 and that "Customers may experience shipping delays and back-orders when demand exceeds production plans and available inventory throughout 2018. Resupply is expected to be continuous and expedited throughout 2018 in response to demand".
GSK has shown no indication they will study the use of this adjuvant vaccine in patients with immune or inflammatory disorders. Thus, the safety of Shingrix in a patient with lupus or inflammatory arthritis (on a DMARD or biologic) remains to be seen.
There are only antecdotal reports of no or limited adverse events when used in rheumatic patients.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.