Scleroderma Digital Ulcers Fail to Respond to Endothelin Antagonist Save
Khanna and coworkers have published the results of the DUAL-1 and DUAL-2 clinical trials (sponsored by Actelion Pharmaceuticals) testing the efficacy macitentan in reducing the number of new digital ulcers in patients with systemic sclerosis. The results appear in the May 10th issue of JAMA.
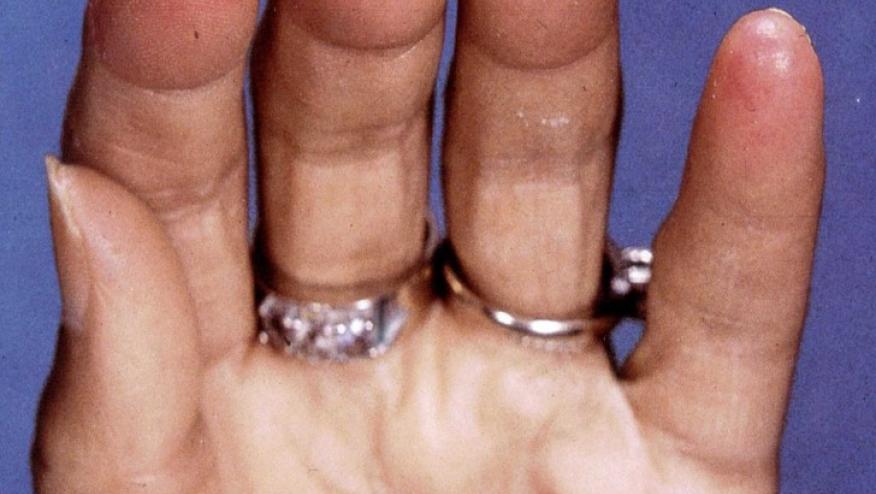
Digital ulcers occur in 35 percent to 68 percent of patients with systemic sclerosis, and are associated with pain, disfigurement, poor quality of life, and disability. Macitentan, an endothelian receptor antagonist, is currently approved for long-term treatment of pulmonary arterial hypertension.
The DUAL-1, DUAL-2 clinical trials treated patients with systemic sclerosis with active digital ulcers and were randomly assigned to receive oral doses of 3 mg of macitentan, 10 mg of macitentan, or placebo once daily and stratified according to number of digital ulcers at baseline. Overall 554 patients were enrolled and 442 completed the trials. Macitentan did not reduce the cumulative number of new digital ulcers over 16 weeks compared with placebo. However, one of the problems of this study was that patients had few new digital ulcers (roughly 1 new ulcer per patient) and their overall digital ulcer condition remained stable over 16 weeks.
Adverse events more frequently seen with macitentan included headache, peripheral edema, skin ulcer, anemia, upper respiratory tract infection, diarrhea, and nasopharyngitis.
The results do not support this agen in the treatment of digital ulcers. Moreover, these results are disappointing given the few effective optoins that exist for such patients. The recent SEDUCE trial failed to prove the benefit of sildenafil in patients with chronic digital ulcerations. (Citation source: http://t.co/SpBWnKeq7L)










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.