Uveitis in JIA: Screen All, Treat Early Save
A European group of experts has formulated consensus-based recommendations for the treatment of juvenile idiopathic arthritis (JIA)-associated uveitis, focusing on screening, monitoring, and treatment of this potentially devastating extra-articular manifestation of JIA.
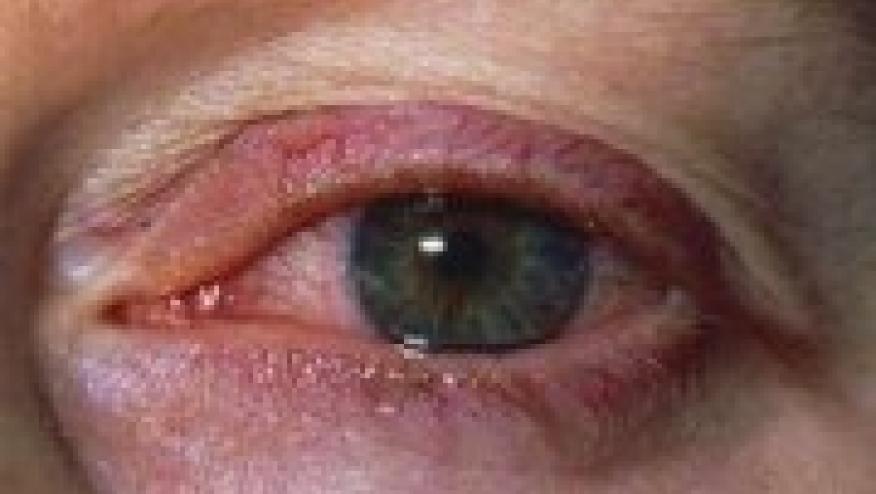
JIA-associated uveitis typically is a chronic relapsing condition that usually begins as anterior uveitis and can persist for several years, according to Athimalaipet V. Ramanan, MD, of the University of Bristol in England, and colleagues. It typically strikes children ages 3 to 7 years, and often is asymptomatic.
Risk factors include early onset arthritis, the oligoarthritis subtype of JIA, and the presence of antinuclear antibodies, and potential complications that can lead to loss of vision include glaucoma, cataracts, macular edema, and band keratopathy, they reported in Annals of the Rheumatic Diseases.
The recommendations were developed by nine experts in pediatric rheumatology and three in ophthalmology.
"With the rapid development of novel therapies for JIA, clear recommendations based on available best evidence and expert opinion (when trial evidence is lacking) will help physicians in the care of patients with JIA-associated uveitis," Ramanan's group wrote.
The first recommendation was that all patients in whom the diagnosis of JIA is being considered should be screened for uveitis. This should be done locally rather than while awaiting referral to a pediatric rheumatology center, because if damage is already present, early and aggressive treatment is needed.
The frequency of follow-up for disease monitoring should be determined by disease severity and in consultation with an expert ophthalmologist. In addition, withdrawal of any systemic immunosuppressant should prompt screening for new or recurrent uveitis every 3 months for at least a year.
For treatment, the experts noted that topical steroids -- preferably prednisolone acetate or dexamethasone -- should be considered the first-line therapy. However, because high-dose topical steroids are associated with the development of cataracts, they suggested that doses no higher than 3 drops per day be used.
Topical and systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have shown no benefit when used as monotherapy, but can be used adjunctively. In one older study, the use of NSAIDs permitted reduction in the dose of steroids.
For patients with persistent ocular inflammation despite topical steroids, or for patients with poor prognostic factors present at baseline, systemic immunosuppression is recommended, with methotrexate as the first-line choice. Those prognostic factors include male sex, glaucoma and cataracts, uveitis present before arthritis, macular edema, and posterior synechia.
Systemic immunosuppressive treatment is also recommended if inactive disease could not be achieved in 3 months of topical steroid therapy or if inflammation increases during steroid taper.
Should methotrexate be ineffective or poorly tolerated, biologic therapy is an option. "A wide range of biological therapies have been investigated in JIA-related uveitis refractory to conventional therapy, with adalimumab [Humira] being the most widely studied," the authors wrote.
Few studies have directly compared the biologics. In one study conducted in Finland, infliximab (Remicade) was more effective than etanercept (Enbrel), and in other studies, adalimumab was superior to infliximab. Moreover, in a small study of patients with severe, persistent uveitis who had previously received infliximab, adalimumab, and rituximab (Rituxan), 14 out of 17 patients responded to golimumab (Simponi). Therefore, the recommendations favor adalimumab as the first biologic, followed by infliximab and then golimumab. Etanercept is not recommended, as it has shown high rates of relapse and flares.
Evidence is also growing suggesting that non-TNF biologics such as abatacept (Orencia) and tocilizumab (Actemra) may have some utility. A trial of tocilizumab in children with uveitis that has not responded to TNF inhibition is underway in the U.K.
The authors furthermore suggested that in cases of a lack of efficacy, consideration be given to testing for the presence of antidrug antibodies and trough levels of the drug. If no antibodies are present and trough levels are low, the dose could be increased or the interval between doses shortened.
The authors also agreed that controlled clinical trials are needed, as are validated outcome measures of factors such as quality of life, disability, visual acuity, and structural complications.
They pointed out that the overall quality of evidence they identified in the literature was low, with only two of 22 recommendations being level 1. "This highlights the need for more research in this clinical setting where a number of new therapies, particularly biological agents, have been introduced in recent years," they stated.
The study was supported by AbbVie.
The authors disclosed relevant relationships with Arthritis Research UK, AbbVie, Pfizer, Novartis, Alimera Sciences, Allergan, Merck Sharp & Dohme, Santen, Xoma, Chugai, Lilly, Medac, Genentech, Bayer, Roche, Bristol-Myers Squibb, UCB, and SOBI.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.