Drug-Induced Dermatomyositis Save
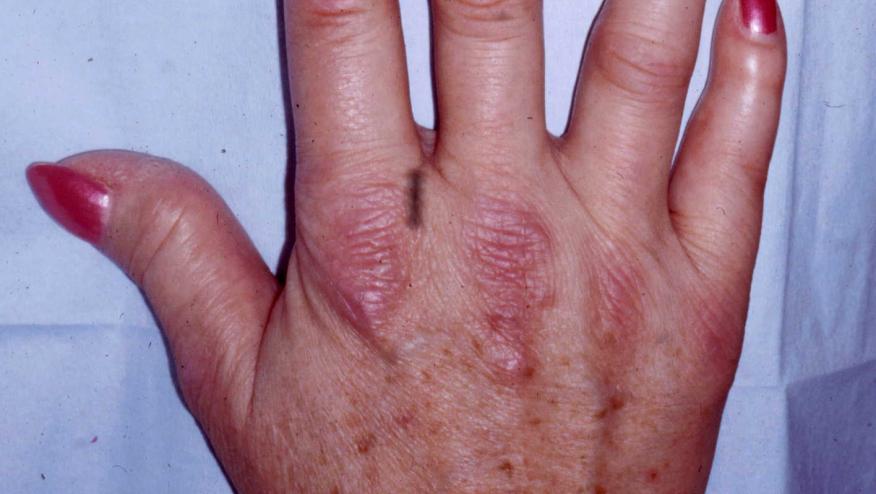
A JAMA Dermatology systematic review of drug-induced dermatomyositis (DM) found the most commonly implicated agents include hydroxyurea, immune checkpoint inhibitors, statins and lipid-lowering agents, penicillamine, and tumor necrosis factor inhibitors.
The systematic reviews and meta-analyses narrowed the search to 134 studies describing 165 cases; 88 patients (53.3%) were female, with a median age of 61 (49-69) years. The median time from drug initiation and drug-induced DM onset was 60 (21-288) days. A history of cancer was reported in 85 cases (52%) - the most common being chronic myelogenous leukemia (22%), melanoma (6%), breast cancer (3%), and polycythemia vera (3%). Fifteen of 166 patients had prior rheumatic disease (11 RA, 3 idiopathic DM, and 2 psoriasis).
Skin manifestations were similar to idiopathy DM, presenting with symmetrical photodistributed erythematous scaly plaques (eg, periorbital erythema, V sign, shawl sign, Gottron papules/sign). Muscle weakness was reported in 44%, and diagnostic tests (when performed) showed electromyography and magnetic resonance imaging to be positive for myopathy in nearly 82%. The frequency of ILD in drug-induced DM appears to be lower compared idiopathic DM (31% vs 42-53%, respectively). The most common autoantibody was a positive antinuclear antibody (31%).
Most common reported cases of drug-induced DM implicated
- hydroxyurea (50 [30%])
- immune checkpoint inhibitors (27 [16%])
- statins (22 [13.3%]), penicillamine (10 [6%])
- tumor necrosis factor inhibitors (10 [6%]).
DM may be idiopathic or drug-induced, with the latter having less clinical weakness, ILD and autoantibodies.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.