Pregnancy Management in Inflammatory Bowel Disease: A Global Consensus Save

Consensus guidance on the management of pregnancy in patients with inflammatory bowel disease (IBD) was simultaneously published in six international journals, including The American Journal of Gastroenterology.
The Global Consensus Consortium is a group of 39 IBD and content experts and 7 patient advocates from 6 continents who convened to review and assess current data and come to an agreement on best practices based on these data. They utilized the GRADE process to review and evaluated the published data and to develop expert opinion and consensus to guide current practice. Recommendations were based on the guiding principle that maternal health best supports infant health and outcomes.
A total of 34 GRADE recommendations and 35 Consensus statements were promulgated on 10 topic areas. Guidelines addressed appropriate counseling and education, drug used during pregnancy in IBD patients.
The guideline is summarized by the infographic below: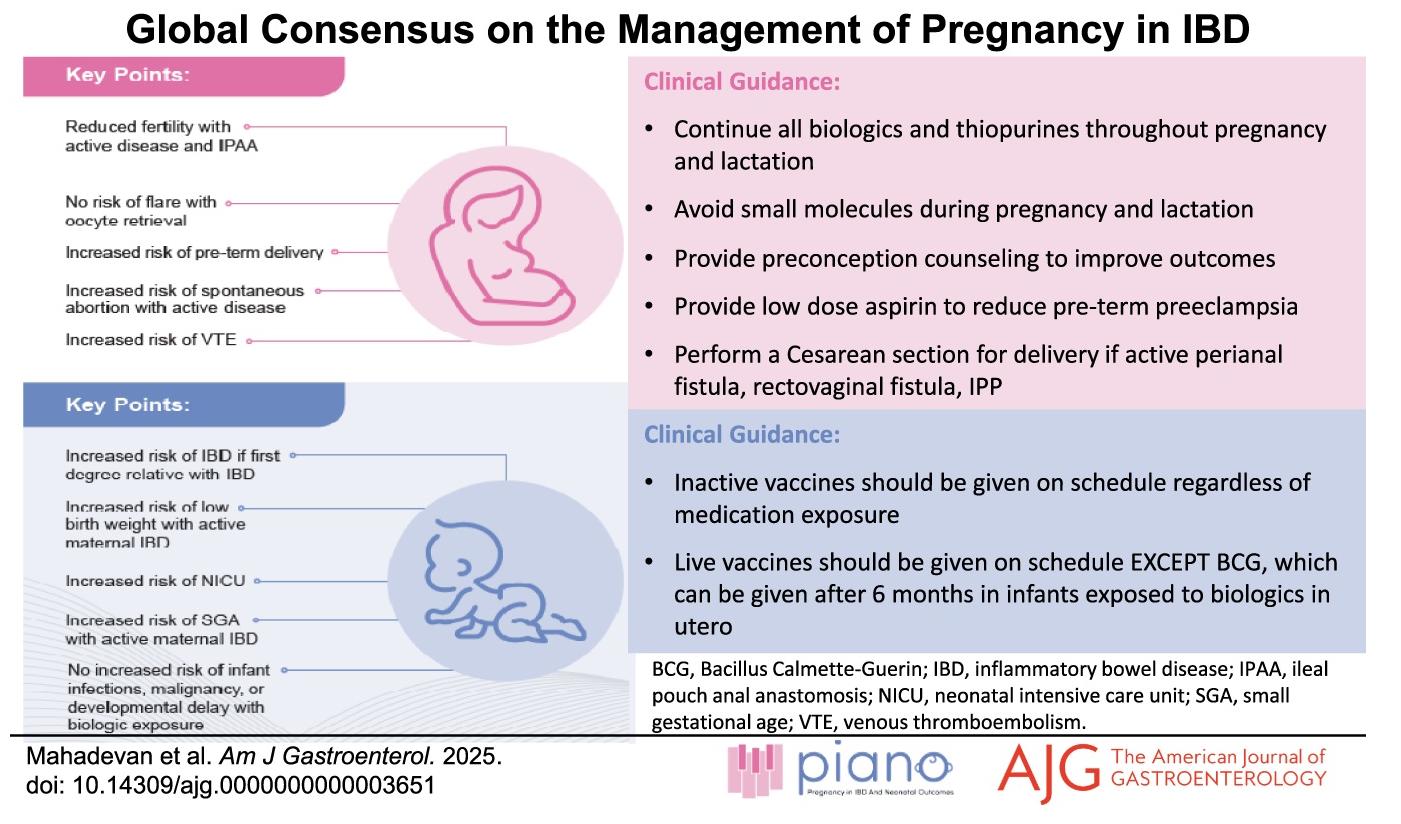
Other (selected) guidance statements relevant to rheumatology patients, comes from these statements pertaining to IBD patients (with strength of recommendation and level of evidence):
- We suggest that pregnant women with IBD take low-dose aspirin by 12–16 weeks gestation to prevent preterm preeclampsia. (Conditional, Low evidence)
- For women with IBD who are pregnant or attempting conception, we recommend continuing maintenance 5-ASA therapy. (Strong Low)
- In women with IBD who are pregnant or attempting conception, we suggest continuing maintenance sulfasalazine therapy. (Conditional Very low)
- In women with IBD who are pregnant, we suggest use of corticosteroid therapy when clinically necessary with appropriate monitoring. (Conditional Low)
- In women with IBD, we recommend discontinuing maintenance methotrexate therapy prior to conception. (Strong Very low)
- In women with IBD who are pregnant or attempting conception, we recommend continuing maintenance anti-TNF therapy throughout pregnancy. (Strong Low)
- In women with IBD who are pregnant or attempting conception, we suggest continuing maintenance combination therapy with an anti-tumor necrosis factor and thiopurine therapy throughout pregnancy. (Conditional Very low)
- In women with IBD who are pregnant or attempting conception, we suggest continuing maintenance vedolizumab therapy throughout pregnancy. (Conditional Low)
- In women with IBD who are pregnant or attempting conception, we suggest continuing maintenance ustekinumab therapy throughout pregnancy. (Conditional Low)
- We recommend breastfeeding as it is not associated with an increased risk of disease exacerbation in women with IBD. (Strong Very low)
- We suggest counseling that infants born to mothers on anti-TNF therapy who breastfeed have no increased risk of infection in the first 12 months of life. (Conditional Very low)
- We suggest counseling that women with IBD as compared with women without IBD have an increased risk of adverse pregnancy outcomes including low birth weight and preterm delivery. (Conditional Very low)
- We suggest counseling that children born to women treated with anti-tumor necrosis factor therapy, ustekinumab, or vedolizumab during pregnancy have no increased risk for early childhood malignancy. (Conditional Very low)
- We suggest counseling that children born to women treated with anti-tumor necrosis factor therapy, ustekinumab, or vedolizumab during pregnancy have no increased risk for early childhood developmental delay. (Conditional Very low)
- Women with IBD who are pregnant and with active disease should start or optimize the same appropriate therapies as in nonpregnant patients, except for thiopurines, methotrexate, JAKis inhibitors, and S1P receptor modulators.
- Women with IBD may initiate or continue calcineurin inhibitors (cyclosporine and tacrolimus) during pregnancy with careful monitoring if there are no viable alternate treatment options available.
- Women with IBD who are pregnant or attempting conception should continue biosimilars to existing biologics.
- Women with IBD who are pregnant or attempting conception should continue anti-interleukin (IL)-23 therapy throughout pregnancy (mirikizumab, risankizumb, guselkumab).
- Women with IBD should discontinue tofacitinib at least 4 weeks prior to conception unless there is no effective alternative therapy to maintain maternal health.
- Women with IBD should discontinue upadacitinib at least 4 weeks prior to conception unless there is no effective alternative therapy to maintain maternal health.
- Women with IBD should discontinue filgotinib at least 4 weeks prior to conception unless there is no effective alternative therapy to maintain maternal health.
- We recommend that inactive vaccines be provided to children born to mothers with IBD on anti-TNF agents (Strong recommendation, very low level of evidence).
- We suggest that live rotavirus vaccine may be provided in children with in utero exposure to biologics (Conditional recommendation, very low level of evidence).
- We recommend that live Bacillus Calmette-Guérin vaccine be avoided in the first 6 months of life in children with in utero exposure to anti-TNF due to risk of disseminated tuberculosis and associated mortality (Strong recommendation, very low level of evidence).
- Inactive vaccines should be given on schedule to infants of women with IBD regardless of in utero IBD medication exposure.
- Live vaccines can be given to infants of mothers breastfeeding while on biologics.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.