IL-23 Inhibitor Performance in Psoriatic Disease Save
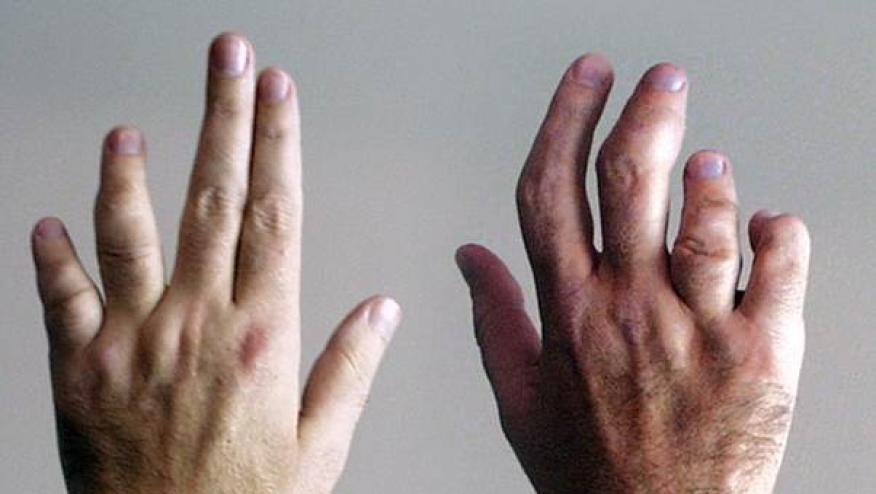
Interleukin-23 (IL-23) inhibitors are approved for the treatment of psoriasis (PsO) and psoriatic arthritis (PsA); a single center real-world observational study shows three commercially available IL-23 inhibitors have high and similar drug survival and effectiveness in difficult-to-treat psoriasis and PsA.
This retrospective analysis included PsO and PsA patients treated with either guselkumab, tildrakizumab, or risankizumab at the Department of Dermatology, Aarhus University Hospital, from June 2018 to July 2021.
A total of 80 patients were included, and 19 patients discontinued IL-23 treatment with a mean treatment duration pf 61.4 weeks. While 95% had previously used ≥1 biologic
One-year drug survival was 81.0%. Two-thirds of patients achieved a Psoriasis Area and Severity Index (PASI) ≤ 2 between weeks 12 - 60. There was no statistically significant difference between the three IL-23 drugs and achieving PASI ≤ 2.
One-quarter (27.5%) had PsA, with 41% achieved complete remission and 36% achieved partial remission.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.