Pegloticase Plus Methotrexate in Refractory Gout - MIRROR Study 12 mos. Results Save
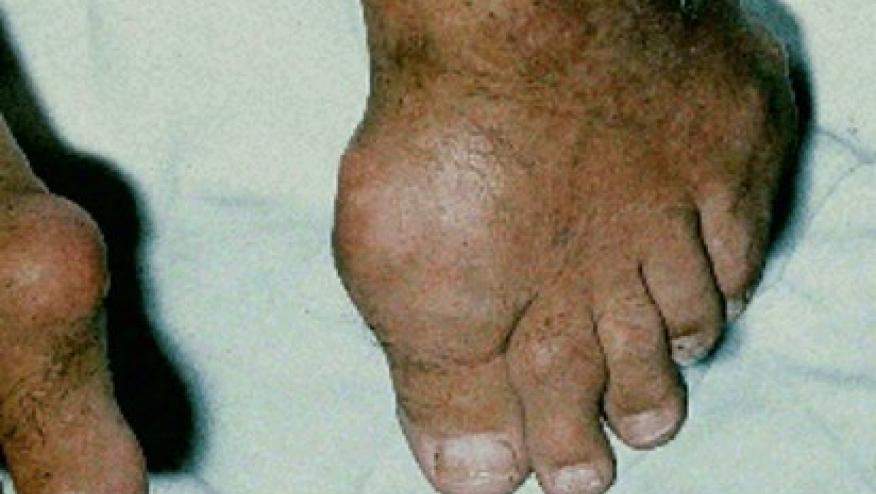
The 12-month safety and efficacy of pegloticase + methotrexate (MTX) in the MIRROR trial shows continued efficacy and tophi resolution after 12 months of therapy.
MIRROR was a randomized, double-blind, placebo-controlled, multicenter, efficacy and safety study of methotrexate (MTX) or placebo (PBO) added to background pegloticase IV to see if MTX would decrease the immunogenicity of PEG and improve clinical outcomes and the duration of PEG therapy in uncontrolled, refractory gout patients.
Patients were treated (2:1) with intravenous pegloticase (8-mg infusion every 2 weeks) plus or minus oral blinded MTX (15 mg/week) for 52 weeks. Efficacy end points included proportion of responders (SU level <6 mg/dl for ≥80% of examined month). Secondary outcomes included resolution of one or more tophi; mean serum uric acid (SU) reduction; and time to SU-monitoring pegloticase discontinuation.
At month 12, response rates were significantly higher in patients co-treated with MTX
- MTX 60.0% [60 of 100]
- Placebo 30.8% [16 of 52]; (P = 0.0003),
MTX coadministration lead to fewer SU discontinuations (22.9% 63.3% on PBO) and more complete resolution of tophi (53.8% versus 31%; P = 0.048),
Pharmacokinetic and immunogenicity findings from 6 mos. persisted to 12 mos. No infusion reactions occurred after 24 weeks.
These long-term data support the use of MTX co-therapy with pegloticase in refractory gout patients.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.