FDA Approves Taltz for Psoriasis Save
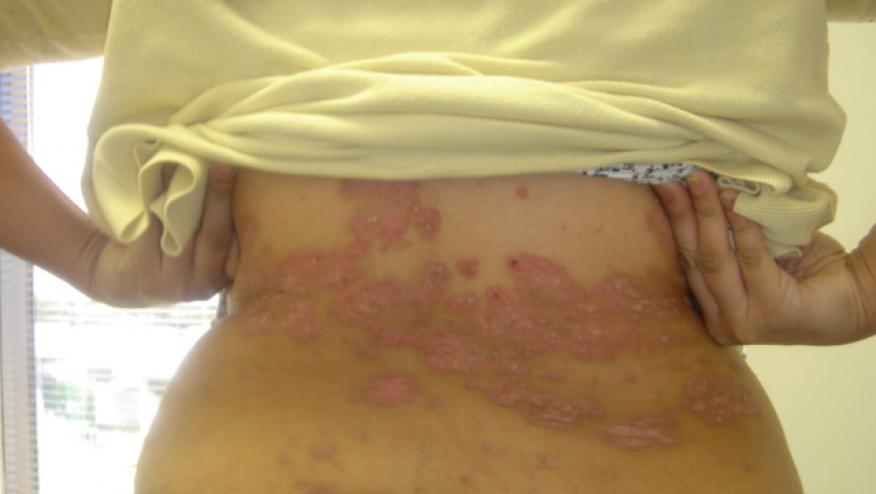
Tuesday the FDA approved the IL-17 inhibitor, Taltz (ixekizumab) for adults with moderate to severe plaque psoriasis. (Citation source http://buff.ly/1py10wd)
Taltz’s is an antibody (ixekizumab) that binds interleukin (IL)-17A. It's safety and efficacy were established in three randomized, placebo-controlled clinical trials involving 3,866 participants with plaque psoriasis who were candidates for systemic or phototherapy therapy.
The results of the 3 pivotal studies (UNCOVER 1-3) showed that Taltz achieved superior clinical responses compared to placebo, with impressive PASI 75 and PASI 90 results. In all three studies, at 12 weeks, 87-90% of patients treated with Taltz saw a significant improvement in PASI 75 scores. In addition, 68-71% achieved PASI 90 responses and 35-42% had complete resolution of their psoriasis plaques (PASI 100, sPGA 0). The most common side effects include upper respiratory infections, injection site reactions and fungal (tinea) infections.
Taltz is the second IL-17 inhibitor to receive approval for psoriasis. The other (Cosentyx or secukinumab) was approved late last year for the same indication.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.