Adalimumab Approved for Uveitis Save
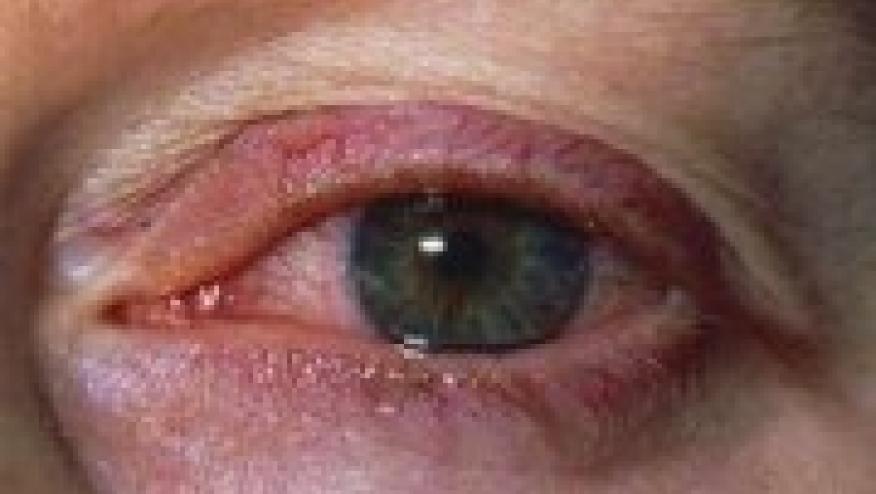
Autoimmune ophthalmic disease is a frequent challenge to rheumatologists from both a diagnostic and therapeutic perspective. Approved therapies are limited and have only been infrequently or comfortably utilized by rheumatologists and autoimmune ophthalmologists.
Yesterday, AbbVie announced that the U.S. Food and Drug Administration (FDA) has approved adalimumab for the treatment of non-infectious intermediate, posterior and panuveitis. This is the 10th indication for adalimumab.
Uveitis trials are very difficult to successfully design and complete and the Abbvie VISUAL clinical trial program composed of several studies demonstrated benefit of adalimumab in patients with non- infectious intermediate, posterior and panuveitis. Although these forms of uveitis are seen as idiopathic entities they frequently are associated with established autoimmune diseases such as Behcet’s, Sarcoidosis, JIA and the HLA B 27 associated diseases. The fact that adalimumab has already been used and in some instances is already approved for some of these illnesses is a further benefit of this approval.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.