Adalimumab Gets FDA Orphan Drug Status for Use in Hidradenitis Suppurativa Save
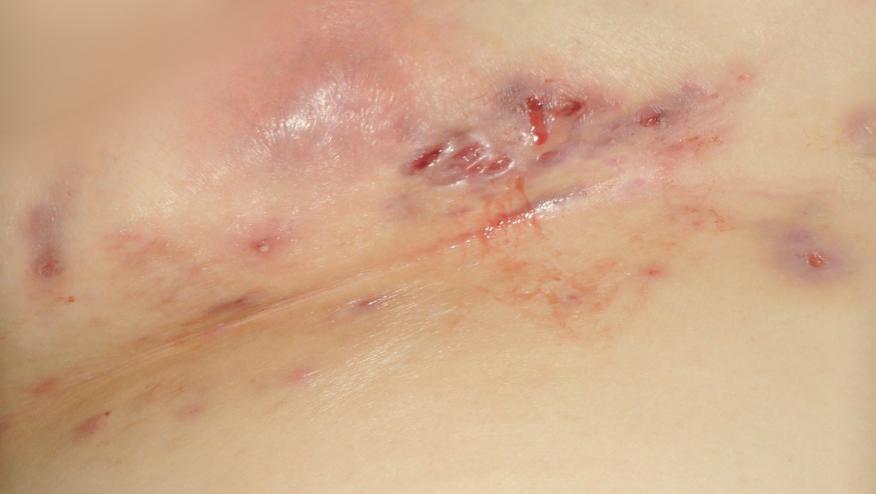
The FDA has granted adalimumab (Humira) orphan drug designation for the investigational treatment of moderate-to-severe hidradenitis suppurativa (HS) (Hurley Stage II and Hurley Stage III disease). HS is a painful, chronic inflammatory skin disease for which there is no known cure and no approved medication. The disease is characterized by inflamed areas typically located around the axilla or groin, between the buttocks and under the breasts. A number of physical signs include painful abscesses and nodules, sinus tracts and scarring.
AbbVie's supplemental Biologic License Application seeking FDA approval for the use of HUMIRA in patients with moderate-to-severe HS is currently under review with the agency. HUMIRA is not currently approved by regulatory authorities for the treatment of HS.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.