Apremilast Reduces Oral Ulcers in Behcet's Syndrome Save
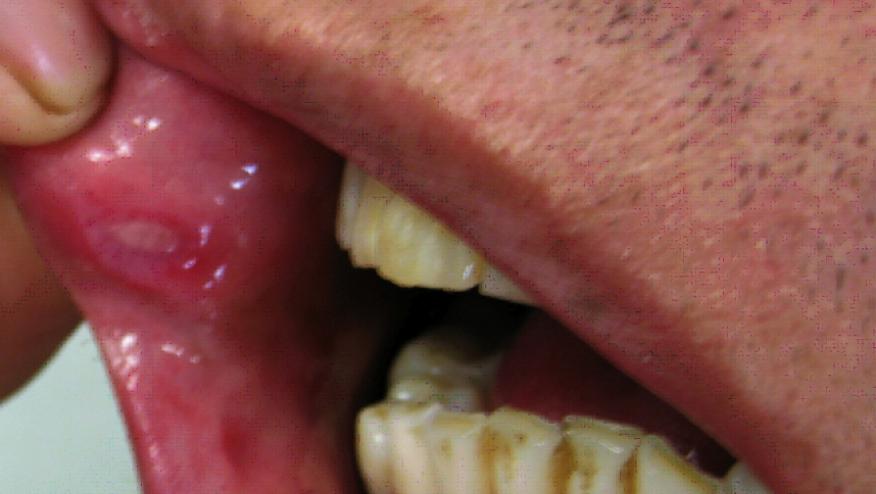
A multinational trial finds a statistically significant number of patients with Behçet's syndrome showed a greater reduction in the number of oral ulcers with apremilast compared with placebo.
The inflammatory condition Behçet's syndrome commonly affects small blood vessels in the mouth, genitals, skin, and eyes. The highest prevalence of Behçet's occurs in northern Turkey, where it affects up to 420 in 100,000 people. This multisystem vasculitis is rarer in northern European countries and the U.S., where it generally affects fewer than one in 100,000 people. The most widespread, and often the first, symptoms of the syndrome are aphthous ulcers, which are often recurrent and may not respond to available therapies.
Note that this multisystem vasculitis generally affects fewer than one in 100,000 people in the U.S., and the most widespread symptoms of the syndrome are aphthous ulcers, which are often recurrent and may not respond to available therapies.
Apremilast is an oral small-molecule phosphodiesterase 4 inhibitor of the release of proinflammatory markers such as interleukin-2, -8, -12, and -17. The drug prevents degradation of cyclic adenosine monophosphate, reducing the release of proinflammatory cytokines and increasing the production of anti-inflammatory mediators. As a regulator of downstream inflammatory signaling, this drug has been used to treat patients with other inflammatory conditions, including psoriasis and psoriatic arthritis.
According to a randomized multinational phase III study, treatment with apremilast led to improvements in active oral ulcers and multiple other outcomes in 207 patients with Behçet's syndrome who had not received any previous biologics. The area under the curve (AUC) for the number of oral ulcers during 12 weeks of treatment -- the primary efficacy endpoint -- was 129.5 for apremilast compared with 222.1 for placebo, reported Gülen Hatemi, MD, of Istanbul University and the Behçet's Disease Research Center in Turkey, and colleagues.
That represented a least-squares mean difference of -92.6 (95% CI -130.6 to -54.6, P<0.001), the researchers wrote in the New England Journal of Medicine.
Previously tested in a phase II study, apremilast decreased the number of oral ulcers, associated pain, and disease activity in Behçet's patients.
In order to examine apremilast's efficacy and safety in a broader cohort of patients, Hatemi and colleagues designed a larger trial that included patients from 53 centers in 10 countries during the years 2014 to 2017.
They randomized patients 1:1 to receive apremilast, 30 mg twice daily, or placebo for 12 weeks, after which time all participants could enter the next 52-week extension phase of the study. The apremilast dose was increased gradually during the first week to reduce the risk of gastrointestinal side effects.
In order to qualify for the study, patients must have had oral ulcers at least three times during the previous year despite treatment with one or more nonbiologic agents, including glucocorticoids, immunosuppressants, colchicine, or thalidomide.
The participants' mean age was 40, 61% were women, and the mean duration of Behçet's was 6.8 years. The mean number of oral ulcers at baseline was 4.1. While 99% of patients had a history of skin lesions, 56% had active skin lesions at baseline, and 90% had genital lesions, among other symptoms.
Patients were not allowed to use colchicine, glucocorticoids, immunosuppressants, and biologic drugs during the placebo-controlled time period through their ninth visit.
Investigators assessed oral ulcers at weeks 0, 1, 2, 4, 6, 8, 10, and 12 during the placebo-controlled trial period.
At week 12, the change in ulcer pain scores for treated patients was -42.7 among those receiving the drug, compared with -18.7 for those taking the placebo, for a least-squares mean difference of -24.1 (95% CI -32.4 to -15.7). At that time point, change from baseline on the Behçet's Syndrome Activity Scale was -19.8 in the apremilast group compared with -8.8 in the placebo group, for a least-squares mean difference of -11 (95% CI -15.6 to -6.4).
The percentage of patients who had a complete response for their oral ulcers at week 12 was 53% in the apremilast group versus 22% in the placebo group, and the median time to complete response was 2.1 weeks versus 8.1 weeks.
The percentage of patients who had no ulcers by week 6 and remained free of ulcers for another 6 weeks was 30% in the apremilast group compared with 5% in the placebo group.
The change from baseline in the Behçet's Disease Quality of Life score was -4.3 versus -1.2, for a least-squares difference of -3.1 (95% CI -4.9 to -1.3).
Among patients who had genital ulcers present at baseline (17 in each group), complete responses were seen at week 12 in 71% of those taking apremilast compared with 41% of those taking placebo; the between-group difference was not significant.
Thus far, 46 patients in the apremilast group and 47 in the placebo group who switched over to apremilast have completed the study.
The safety analysis included both the 12-week controlled phase plus the overall apremilast exposure period. During the placebo-controlled phase, more patients in the active treatment group reported adverse events:
- Diarrhea, 41% vs 20%
- Nausea, 19% vs 11%
- Headache, 14% vs 11%
- Upper respiratory tract infection, 12% vs 5%
Two patients in the placebo group had flares of uveitis, while none of the patients in the apremilast group developed uveitis.
In the longer apremilast exposure period, the incidence rates of diarrhea were 49.7 per 100 patient-years for the placebo/apremilast group and 89.7 per 100 patient-years in the original apremilast group, while nausea was reported by 16.9 and 32.0 per 100 patient-years in the two groups, respectively.
Overall, 94% of patients in the treatment group and 95% of the placebo group remained on the study.
A limitation of the study, the researchers said, was that it was not designed to assess the effects of apremilast on mucocutaneous symptoms other than the oral ulcers.
Source Reference: New England Journal of Medicine 2019; DOI: 10.1056/NEJMoa1816594










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.