2025 BSR Guideline for Treatment of Axial Spondyloarthritis Save

The British Society of Rheumatology has published its 2025 Guidelines for the Treatment of Axial Spondyloarthritis (axSpA); addressing axial and extra-musculoskeletal manifestations including acute anterior uveitis, psoriasis and IBD. They address the effectiveness and safety of targeted therapies; switching, combining, tapering or withdrawing targeted therapies; and treating to target. The guideline applies only to adults with axSpA. Biologic and targeted synthetic DMARDs (b/tsDMARDs) are referred to as “targeted therapies”.
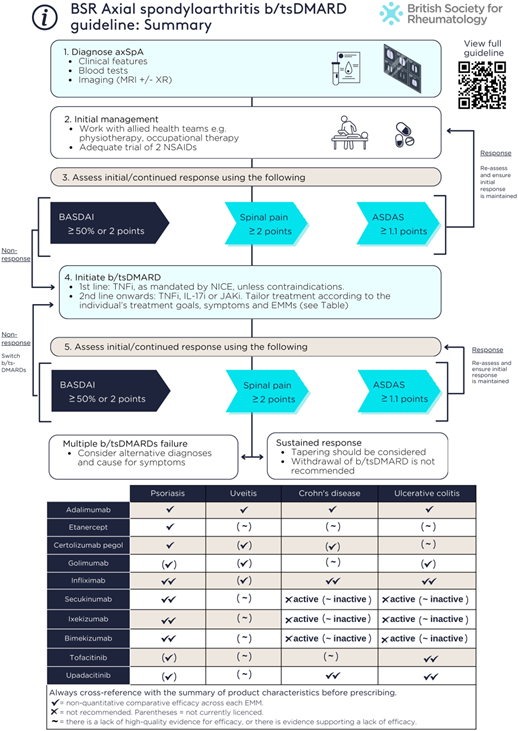
The guideline includes 3 overarching principles and 15 guideline recommendations.
Overarching principles
- The primary goal of treatment is to enable patients, optimize quality of life, prevent structural damage, and preserve physical function, work productivity and social participation
- Shared management decisions should be developed between patient and provider
- Management should involve a multidisciplinary team, a holistic approach and use both pharmacological and non-pharmacological interventions
Recommendations
- TNF, IL-17 or JAK inhibitors are recommended for people with active axSpA who have not responded adequately to non-pharmacological and conventional pharmacological management
- Active disease should be determined by the treating clinician in the context of verified diagnosis and inflammatory disease activity
- Response to targeted therapies should be assessed using validated indices (e.g. ASDAS, BASDAI, spinal pain) 3–4 months after initiation, and every 6–12 months if treatment is continued.
- The absence of response to targeted therapies should prompt reassessment of the diagnosis and the extent of inflammatory disease activity
- An alternative targeted therapy is recommended for individuals with active disease who cannot tolerate, do not respond to, or lose response to the initial targeted therapy
- In the presence of moderate-to-severe or recurrent uveitis, a monoclonal TNFi is preferred over therapies with other mechanisms of action
- A history of inactive uveitis is not an absolute contraindication to therapies with other mechanisms of action
- If new uveitis develops in the context of well-controlled axSpA, decisions to change treatment should be made with an ophthalmologist where possible, taking into account the severity and/or frequency of uveitis flares and response to topical steroid
- IL-17 and monoclonal TNFi are preferred in the presence of extensive psoriasis (e.g. >10% body surface area) or severe localized psoriasis at sites associated with high functional impairment or impact (e.g. face, scalp, palms, soles, flexures, genital or nails), ideally in conjunction with a dermatologist
- Individuals with unexplained lower gastrointestinal symptoms should be assessed by a gastroenterologist, ideally before commencing targeted therapies
- In the presence of active IBD, monoclonal TNFi or JAKi are preferred; IL-17 inhibitors should not be commenced
- A history of inactive IBD is not an absolute contraindication to IL-17 inhibitors or etanercept
- Treatment should aim to achieve predefined targets agreed upon with the individual living with axSpA, using individualized therapy adjustments that consider comorbidities and inflammatory disease activity
- Tapering of targeted therapies should be considered for individuals who have achieved sustained remission
- Withdrawal of targeted therapies in the context of sustained remission is not recommended
The areas the guideline does not cover
- NSAIDs, glucocorticoids and conventional synthetic DMARDs.
- Treatment of enthesitis/spondylitis-related juvenile idiopathic arthritis.
- Axial disease in psoriatic arthritis.
- Safety of targeted therapies or their use in pregnancy
- Health economic considerations.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.