Herpes Zoster Vaccination in Immunocompromised Adults Save
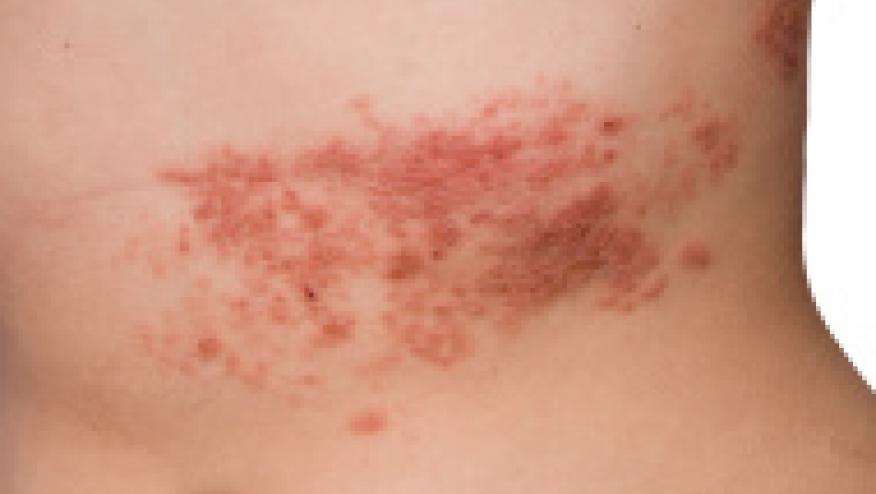
The CDC's Advisory Committee on Immunization Practices (ACIP) recently recommended and approved two doses of the recombinant zoster vaccine (RZV, AKA Shingrix) for prevention of herpes zoster and complications in immunodeficient or immunosuppressed adults aged ≥19 years.
The previously advocated live virus H. zoster vaccine (Zostavax) is no longer commercially available and the newer RZV has come to dominate the market. RZV development did not include autoimmune and immunocompromised individuals, but they have been using the newer, non-live, recombinant vaccine nonetheless. On July 23, 2021, the FDA expanded the indication for use of recombinant zoster vaccine (RZV) to include immunodeficient or immunosuppressed adults.
This recommendation adds to the safety profile of RZV when given to Immunocompromised persons, who are at a higher risk for herpes zoster and related complications. RZV has moderate to high vaccine efficacy and an acceptable safety profile.
Shingrix (RZV) is a two dose (0.5 mL each ) subunit vaccine containing recombinant glycoprotein E in combination with an adjuvant (AS01B), that was approved by the FDA in 2017 for the prevention of herpes zoster for adults aged ≥50 years.
Between 2017 and October 2021, the ACIP Herpes Zoster Work Group participated in monthly or bimonthly teleconferences to review herpes zoster epidemiology and evidence for the efficacy and safety of RZV in immunocompromised adults. They had and ongoing evidence based evaluation (GRADE) of the possible benefits (prevention of herpes zoster, postherpetic neuralgia, and herpes zoster-related hospitalizations) and harms (serious adverse events [SAEs], immune-mediated disease, graft-versus-host-disease, graft rejection, and reactogenicity) associated with RZV.
The RZV vaccine efficacy was studied in 5 studies in four immunocompromised groups, with estimates of vaccine efficacy of 68.2% in autologous hematopoietic cell transplant recipients, 87.2% and 90.5% in hematologic malignancies and potential immune-mediated diseases, respectively.
SAEs were evaluated in seven studies in six immunocompromised groups with comparable SAE rates between RZV and placebo recipients (risk ratios ranged from 0.79 to 1.99).
RZV has the potential to prevent considerable herpes zoster incidence and related complications. It is recommended for use in immunocompromised adults aged ≥19 years with the following Clinical Guidance:
- Dosing schedule. Two RZV doses are necessary, regardless of previous history of herpes zoster or previous receipt of zoster vaccine live. The second RZV dose should typically be given 2–6 months after the first; for persons who are or will be immunodeficient or immunosuppressed and who would benefit from a shorter vaccination schedule, the second dose can be administered 1–2 months after the first (2). If the second RZV dose is given sooner than 4 weeks after the first, a valid second dose should be repeated at least 4 weeks after the dose given too early. The vaccine series does not need to be restarted if more than 6 months have elapsed since the first dose.
- Timing of vaccination. When possible, patients should be vaccinated before becoming immunosuppressed. Otherwise, providers should consider timing vaccination when the immune response is likely to be most robust (i.e., during periods of lower immunosuppression and stable disease). RZV may be administered to patients who previously received varicella vaccine. RZV is not a live virus vaccine; therefore, RZV may be administered while patients are taking antiviral medications.
- Coadministration with other vaccines. Recombinant and adjuvanted vaccines, such as RZV, can be administered concomitantly, at different anatomic sites, with other adult vaccines, including COVID-19 vaccines (17). Concomitant administration of RZV with other adult vaccines has been studied, and there was no evidence for interference in the immune response to either vaccine or of safety concerns. Coadministration of RZV with adjuvanted influenza vaccine (Fluad) and COVID-19 vaccines is being studied.
- Counseling for reactogenicity. Before vaccination, providers should counsel patients about expected local and systemic reactogenicity, including grade 3 reactions. It is generally not recommended to take antipyretic or analgesic medications prophylactically before vaccination; however, antipyretic or analgesic medications may be taken for the treatment of postvaccination local or systemic symptoms. Patients should be encouraged to complete the series even if they experienced a (nonanaphylactic) grade 1–3 reaction after receipt of the first RZV dose.
Special Populations
- Persons with a history of herpes zoster. Herpes zoster can recur. Persons with a history of herpes zoster should receive RZV.
- Persons with no documented history of varicella, varicella vaccination, or herpes zoster. Persons who have neither experienced varicella nor received varicella vaccine are not at risk for herpes zoster. More than 99% of Americans born before 1980 have had varicella. Children and adolescents who have received live-attenuated varicella vaccines are at lower risk for herpes zoster than are those who experienced varicella. RZV is not indicated and has not been studied for the prevention of varicella. For immunocompromised persons, evidence of immunity to varicella (confirming need for RZV) includes documented receipt of 2 doses of varicella vaccine, laboratory evidence of immunity or laboratory confirmation of disease, or diagnosis or verification of a history of varicella or herpes zoster by a health care provider. For immunocompromised adults with no documented history of varicella, varicella vaccination, or herpes zoster, providers should refer to the ACIP varicella vaccine recommendations for further guidance, including postexposure prophylaxis guidance.
- Pregnancy. There is currently no ACIP recommendation for RZV use in pregnancy; therefore, providers should consider delaying RZV until after pregnancy. There is no recommendation for pregnancy testing before vaccination.
- Breastfeeding. Recombinant vaccines such as RZV pose no known risk to mothers who are breastfeeding or to their infants (17). Clinicians may consider vaccination without regard to breastfeeding status if RZV is otherwise indicated.
Contraindications
- Allergy. RZV should not be administered to persons with a history of severe allergic reaction, such as anaphylaxis, to any component of this vaccine.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.